Epigenetic Modifications as Novel Biomarkers for Diagnosis, Prognosis, and Therapeutic Targeting in Thyroid, Pancreas, and Lung Neuroendocrine Tumors
- PMID: 40283452
- PMCID: PMC12027509
- DOI: 10.3390/jcm14082622
Epigenetic Modifications as Novel Biomarkers for Diagnosis, Prognosis, and Therapeutic Targeting in Thyroid, Pancreas, and Lung Neuroendocrine Tumors
Abstract
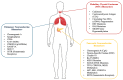
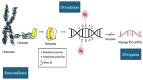
Neuroendocrine neoplasms (NENs) comprise a heterogeneous tumor group arising from neuroendocrine cells, commonly originating in the gastroenteropancreatic tract and bronchopulmonary system. Their incidence has risen significantly, owing to improved diagnostic techniques and increased clinical recognition. While previous reviews have explored the molecular and genetic basis of NENs, limited attention has been given to the role of epigenetic modifications, particularly DNA methylation, in tumorigenesis and disease progression. This review focuses on lung, pancreas, and thyroid well-differentiated neuroendocrine tumors (NETs), highlighting epigenetic mechanisms, particularly DNA methylation, as promising biomarkers for early diagnosis and risk stratification. Aberrant DNA methylation can silence key tumor suppressor genes, including RASSF1A and CDKN2A, thereby promoting tumorigenesis. Integrating DNA methylation profiles with conventional biomarkers such as chromogranin A (CgA) may enhance diagnostic accuracy and inform therapeutic strategies. Emerging epigenetic therapies offer potential avenues for personalized treatment based on molecular profiling. Unlike prior reviews that broadly cover genetic and epigenetic changes in NENs, this review uniquely emphasizes the translational potential of epigenetic biomarkers in clinical practice. By synthesizing recent findings and evaluating their clinical implications, we aim to bridge the gap between molecular research and practical applications in diagnosis, prognosis, and therapy.
Keywords: DNA methylation; epigenetic alterations; neuroendocrine neoplasms; well-differentiated neuroendocrine tumors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Yao J.C., Hassan M.M., Phan A.T., Dagohoy C.G., Leary C.C., Mares J.E., Abdalla E.K., Fleming J.B., Vauthey J.-N., Rashid A., et al. One Hundred Years After “Carcinoid”: Epidemiology of and Prognostic Factors for Neuroendocrine Tumors in 35,825 Cases in the United States. J. Clin. Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

