Pharmacokinetic Characterization of Labetalol in Pregnancy (The CLIP Study): A Prospective Observational Longitudinal Pharmacokinetic/Pharmacodynamic Cohort Study During Pregnancy and Postpartum
- PMID: 40283622
- PMCID: PMC12028293
- DOI: 10.3390/jcm14082793
Pharmacokinetic Characterization of Labetalol in Pregnancy (The CLIP Study): A Prospective Observational Longitudinal Pharmacokinetic/Pharmacodynamic Cohort Study During Pregnancy and Postpartum
Abstract
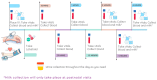
Background/Objectives: Hypertensive disorders of pregnancy are a leading cause of pregnancy-related deaths in the United States, accounting for 7% of maternal mortality. Labetalol and nifedipine are the first-line drugs for the management of hypertension in pregnancy, but there are little data guiding the choice of one drug over the other. The current pilot longitudinal study aims to characterize the pharmacokinetics (PK) and pharmacodynamics (PD) of labetalol stereoisomers throughout pregnancy and postpartum. Methods: This is a single-center clinical study recruiting up to 40 pregnant individuals ≥ 18 years of age at the time of enrollment, taking labetalol as per the standard of care. The exclusion criteria include any pathophysiology impacting the PK of labetalol, e.g., liver failure. Maternal plasma, urine, amniotic fluid, cord blood, and breast milk will be collected, and labetalol stereoisomers will be measured using a validated LC-MS/MS assay. Heart rate and blood pressure will be measured as the PD endpoints. These may be assessed throughout a participant's dosing interval at scheduled PK study visits, which will occur every 6-10 weeks during pregnancy, at delivery, during the 1st week postpartum, and up to 20 weeks postpartum. The primary aim is to characterize the PK and PD of labetalol during pregnancy and in the postpartum period. The secondary aim is to determine the extent of breast milk excretion of and infant exposure to labetalol from breast milk. The data will be analyzed using population PK modeling to evaluate the PK/PD relationship and ultimately develop trimester-specific dosing recommendations. Results/Conclusions: To our knowledge, this is the first study aiming to characterize the PK of labetalol stereoisomers across pregnancy and postpartum, utilizing individual stereoisomer data to evaluate the PK/PD relationship, and collecting postpartum samples, including breast milk, to model infant exposure to labetalol through breast milk. This study will provide important PK/PD data and knowledge which will be combined with large multi-center clinical trial data to develop trimester-specific dosing regimens for anti-hypertensive agents.
Keywords: chiral LC-MS/MS; hypertensive disorders of pregnancy; labetalol; population PK/PD model; protocol.
Conflict of interest statement
All the authors declare there are no financial or personal relationships between themselves or others that might bias their work. The funders were not involved in this study’s design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Figures

Similar articles
-
Incentives for increasing prenatal care use by women in order to improve maternal and neonatal outcomes.Cochrane Database Syst Rev. 2015 Dec 15;2015(12):CD009916. doi: 10.1002/14651858.CD009916.pub2. Cochrane Database Syst Rev. 2015. PMID: 26671418 Free PMC article.
-
Iodine supplementation for women during the preconception, pregnancy and postpartum period.Cochrane Database Syst Rev. 2017 Mar 5;3(3):CD011761. doi: 10.1002/14651858.CD011761.pub2. Cochrane Database Syst Rev. 2017. PMID: 28260263 Free PMC article.
-
Methods for blood loss estimation after vaginal birth.Cochrane Database Syst Rev. 2018 Sep 13;9(9):CD010980. doi: 10.1002/14651858.CD010980.pub2. Cochrane Database Syst Rev. 2018. PMID: 30211952 Free PMC article.
-
Epidural therapy for the treatment of severe pre-eclampsia in non labouring women.Cochrane Database Syst Rev. 2017 Nov 28;11(11):CD009540. doi: 10.1002/14651858.CD009540.pub2. Cochrane Database Syst Rev. 2017. PMID: 29181841 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Cameron N.A., Everitt I., Seegmiller L.E., Yee L.M., Grobman W.A., Khan S.S. Trends in the Incidence of New-Onset Hypertensive Disorders of Pregnancy among Rural and Urban Areas in the United States, 2007 to 2019. J. Am. Heart Assoc. 2022;11:e023791. doi: 10.1161/JAHA.121.023791. - DOI - PMC - PubMed
-
- Vasconcellos D. CDC Press Release: Hypertensive Disorders in Pregnancy Affect 1 in 7 Hospital Deliveries. Neonatology Today. May 1, 2022.
-
- Petersen E.E., Davis N.L., Goodman D., Cox S., Mayes N., Johnston E., Syverson C., Seed K., Shapiro-Mendoza C.K., Callaghan W.M., et al. Vital Signs: Pregnancy-Related Deaths, United States, 2011–2015, and Strategies for Prevention, 13 States, 2013–2017. MMWR Morb. Mortal. Wkly. Rep. 2019;68:423–429. doi: 10.15585/mmwr.mm6818e1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

