Leucine-Rich Alpha-2 Glycoprotein 1 as a Biomarker for Evaluation of Inflammatory Bowel Disease Activity in Children
- PMID: 40283634
- PMCID: PMC12028201
- DOI: 10.3390/jcm14082803
Leucine-Rich Alpha-2 Glycoprotein 1 as a Biomarker for Evaluation of Inflammatory Bowel Disease Activity in Children
Abstract
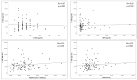
Background: Leucine rich α-2 glycoprotein (LRG) is a glycoprotein that is an acute-phase protein produced by neutrophils, macrophages, hepatocytes, and intestinal epithelial cells. This study aimed to determine the serum LRG (s-LRG) and urine LRG (u-LRG) expression levels in children with inflammatory bowel disease (IBD) and evaluated their correlation with clinical disease activity, other inflammatory markers, laboratory results, and endoscopic activity scoring. Methods: This prospective observational study was conducted at a tertiary centre and included children aged 2-18 years with IBD. Clinic activity scoring was used to assess clinical disease activity. Haemoglobin levels, platelet counts, albumin, C-reactive protein, and erythrocyte sedimentation rate were analysed in the blood sample. LRG levels were measured in both blood and urine samples. The endoscopic assessment was scored according to the simple endoscopic score and Mayo endoscopic score. Serum and urine LRG levels were measured using commercial enzyme-linked immunosorbent assay kits. Disease activation was defined based on clinical activity scoring, laboratory results, and endoscopic evaluation. The results were compared between the active IBD and remission groups. Results: Forty-two (50%) patients with active IBD and forty-two (50%) patients in remission were included in this study. The serum levels of LRG were elevated in the patients with active IBD compared with the levels in the patients with IBD in remission (p = 0.020). However, there was no difference in the u-LRG level between the two groups (p = 0.407). In patients with IBD, positive correlations were observed between s-LRG, platelet count, C-reactive protein (CRP), and the erythrocyte sedimentation rate. The serum LRG was negatively correlated with albumin and haemoglobin levels. Urine LRG was not correlated with s-LRG in any patients with IBD included or in patients with active IBD. The cutoff value for s- LRG (77.03 μg/mL) had a sensitivity and specificity of 40.4% (95% CI 25.6-56.7%) and 88.1% (95% CI 74.3-96.0%), respectively. It was found that s-LRG was a more significant parameter than CRP in predicting disease activation. Conclusions: This prospective study demonstrated that the s-LRG level is a useful biomarker for predicting disease activation in children with IBD and appears to be a more significant parameter than the CRP level. However, the u-LRG level is not effective in predicting disease activation in children with IBD.
Keywords: biomarker; inflammatory bowel disease; leucine-rich alpha-2 glycoprotein 1.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Leucine-Rich Alpha-2 Glycoprotein Is a Reliable Serum Biomarker for Evaluating Clinical and Endoscopic Disease Activity in Inflammatory Bowel Disease.Inflamm Bowel Dis. 2023 Sep 1;29(9):1399-1408. doi: 10.1093/ibd/izac230. Inflamm Bowel Dis. 2023. PMID: 36334015
-
Serum leucine-rich alpha-2 glycoprotein and calprotectin in children with inflammatory bowel disease: A multicenter study in Japan.J Gastroenterol Hepatol. 2023 Jul;38(7):1131-1139. doi: 10.1111/jgh.16166. Epub 2023 Mar 16. J Gastroenterol Hepatol. 2023. PMID: 36880154
-
Leucine-Rich Alpha-2 Glycoprotein in Monitoring Disease Activity and Intestinal Stenosis in Inflammatory Bowel Disease.Tohoku J Exp Med. 2022 Jul 16;257(4):301-308. doi: 10.1620/tjem.2022.J042. Epub 2022 May 20. Tohoku J Exp Med. 2022. PMID: 35598974
-
Leucine-rich Alpha-2 Glycoprotein is a Serum Biomarker of Mucosal Healing in Ulcerative Colitis.J Crohns Colitis. 2017 Jan;11(1):84-91. doi: 10.1093/ecco-jcc/jjw132. Epub 2016 Jul 27. J Crohns Colitis. 2017. PMID: 27466171 Free PMC article.
-
Positioning and Usefulness of Biomarkers in Inflammatory Bowel Disease.Digestion. 2023;104(1):30-41. doi: 10.1159/000527846. Epub 2022 Nov 18. Digestion. 2023. PMID: 36404714 Free PMC article. Review.
Cited by
-
Neutrophils and NETs in Pathophysiology and Treatment of Inflammatory Bowel Disease.Int J Mol Sci. 2025 Jul 23;26(15):7098. doi: 10.3390/ijms26157098. Int J Mol Sci. 2025. PMID: 40806230 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

