Overview of Metformin and Neurodegeneration: A Comprehensive Review
- PMID: 40283923
- PMCID: PMC12030719
- DOI: 10.3390/ph18040486
Overview of Metformin and Neurodegeneration: A Comprehensive Review
Abstract
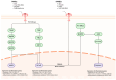
This comprehensive review examines the therapeutic potential of metformin, a well-established diabetes medication, in treating neurodegenerative disorders. Originally used as a first-line treatment for type 2 diabetes, recent studies have begun investigating metformin's effects beyond metabolic disorders, particularly its neuroprotective capabilities against conditions like Parkinson's disease, Alzheimer's disease, Huntington's disease, and multiple sclerosis. Key findings demonstrate that metformin's neuroprotective effects operate through multiple pathways: AMPK activation enhancing cellular energy metabolism and autophagy; upregulation of antioxidant defenses; suppression of inflammation; inhibition of protein aggregation; and improvement of mitochondrial function. These mechanisms collectively address common pathological features in neurodegeneration and neuroinflammation, including oxidative stress, protein accumulation, and mitochondrial dysfunction. Clinical and preclinical evidence supporting metformin's association with improved cognitive performance, reduced risk of dementia, and modulation of pathological hallmarks of neurodegenerative diseases is critically evaluated. While metformin shows promise as a therapeutic agent, this review emphasizes the need for further investigation to fully understand its mechanisms and optimal therapeutic applications in neurodegenerative diseases.
Keywords: metformin; neurodegeneration; type 2 diabetes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Werner E.A., Bell J. CCXIV.—The preparation of methylguanidine, and of ββ-dimethylguanidine by the interaction of dicyanodiamide, and methylammonium and dimethylammonium chlorides respectively. J. Chem. Soc. Trans. 1922;121:1790–1794. doi: 10.1039/ct9222101790. - DOI
Publication types
LinkOut - more resources
Full Text Sources

