Synthesis, Antitumor Activities, and Apoptosis-Inducing Activities of Schiff's Bases Incorporating Imidazolidine-2,4-dione Scaffold: Molecular Docking Studies and Enzymatic Inhibition Activities
- PMID: 40283934
- PMCID: PMC12030650
- DOI: 10.3390/ph18040496
Synthesis, Antitumor Activities, and Apoptosis-Inducing Activities of Schiff's Bases Incorporating Imidazolidine-2,4-dione Scaffold: Molecular Docking Studies and Enzymatic Inhibition Activities
Abstract
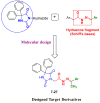
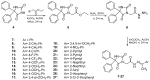
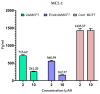
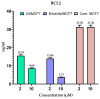
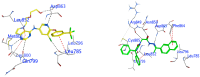
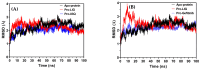
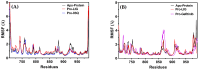
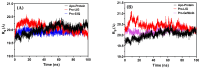
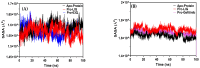
Background/Objective: Cancer is the leading cause of death worldwide despite the diversity of antitumor therapies, which highlights the necessity to explore new anticancer agents. Methods: We synthesized 5,5-diphenylhydantoin derivatives including Schiff's bases 7-27 and evaluated their cytotoxicity via the MTT assay. Enzymatic inhibition assays, cell cycle and apoptosis analyses, and molecular docking studies were also conducted. Results: Derivative 24 demonstrated the highest cytotoxic activity, with IC50 values of 12.83 ± 0.9 μM, 9.07 ± 0.8 μM, and 4.92 ± 0.3 μM against the cell lines HCT-116, HePG-2, and MCF-7, respectively. Compounds 10, 13, and 21 showed potent antitumor activities versus the examined cell lines (average IC50 = 13.2, 14.5, and 13.1 μM), respectively; moreover, these compounds also demonstrated promising EGFR and HER2 inhibitory activities, with IC50 values in the range 0.28-1.61 µM. Derivative 24 displayed the highest EGFR and HER2 inhibitory activity values (IC50 = 0.07 and 0.04 µM), respectively, which were close to those of the reference drugs erlotinib and lapatinib. Therefore, compound 24 was selected for further examinations and exhibited an inducing effect on apoptosis via diminishing the anti-apoptotic protein levels of BCL-2 (8.598 ± 0.29 ng/mL) and MCL-1 (261.20 ± 8.97 pg/mL) and promoting cell cycle arrest at the G2/M phase (33.46%). The binding relationships between compound 24 and the active sites of EGFR and HER2, which are similar to the co-crystallized inhibitors, were investigated using a molecular docking approach. Conclusions: These findings provide insights into the potential anticancer activities of the synthesized derivatives for further optimization to achieve therapeutic use.
Keywords: 5,5-diphenylacetophenone; EGFR2; HER2; molecular docking.
Conflict of interest statement
The authors state that there are no known financial conflicts or interpersonal connections that could have influenced the work published in this study.
Figures
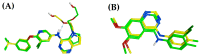

Similar articles
-
Synthesis of novel spirochromane incorporating Schiff's bases, potential antiproliferative activity, and dual EGFR/HER2 inhibition: Cell cycle analysis and in silico study.Saudi Pharm J. 2023 Nov;31(11):101803. doi: 10.1016/j.jsps.2023.101803. Epub 2023 Sep 28. Saudi Pharm J. 2023. PMID: 37860686 Free PMC article.
-
Synthesis, enzyme inhibition assay, and molecular modeling study of novel pyrazolines linked to 4-methylsulfonylphenyl scaffold: antitumor activity and cell cycle analysis.RSC Adv. 2024 Jul 12;14(31):22132-22146. doi: 10.1039/d4ra03902e. eCollection 2024 Jul 12. RSC Adv. 2024. PMID: 39005246 Free PMC article.
-
Design, Synthesis, biological Evaluation, and molecular docking studies of novel Pyrazolo[3,4-d]Pyrimidine derivative scaffolds as potent EGFR inhibitors and cell apoptosis inducers.Bioorg Chem. 2021 Nov;116:105325. doi: 10.1016/j.bioorg.2021.105325. Epub 2021 Sep 4. Bioorg Chem. 2021. PMID: 34507234
-
Novel benzothiazole-based dual VEGFR-2/EGFR inhibitors targeting breast and liver cancers: Synthesis, cytotoxic activity, QSAR and molecular docking studies.Bioorg Med Chem Lett. 2022 Feb 15;58:128529. doi: 10.1016/j.bmcl.2022.128529. Epub 2022 Jan 7. Bioorg Med Chem Lett. 2022. PMID: 35007724 Review.
-
2-Anilinopyrimidine derivatives: Design, synthesis, in vitro anti-proliferative activity, EGFR and ARO inhibitory activity, cell cycle analysis and molecular docking study.Bioorg Chem. 2020 Jun;99:103798. doi: 10.1016/j.bioorg.2020.103798. Epub 2020 Mar 29. Bioorg Chem. 2020. PMID: 32247112 Review.
References
-
- Yahya E.B., Alqadhi A.M. Recent trends in cancer therapy: A review on the current state of gene delivery. Life Sci. 2021;269:119087. - PubMed
-
- Senwar K.R., Reddy T.S., Thummuri D., Sharma P., Naidu V., Srinivasulu G., Shankaraiah N. Design, synthesis and apoptosis inducing effect of novel (Z)-3-(3′-methoxy-4′-(2-amino-2-oxoethoxy)-benzylidene) indolin-2-ones as potential antitumour agents. Eur. Med. Chem. 2016;118:34–46. - PubMed
-
- Iwaloye O., Ottu P.O., Olawale F., Babalola O.O., Elekofehinti O.O., Kikiowo B., Adegboyega A.E., Ogbonna H.N., Adeboboye C.F., Folorunso I.M., et al. Computer-aided drug design in anti-cancer drug discovery: What have we learnt and what is the way forward? Inform. Med. Unlocked. 2023;41:101332. doi: 10.1016/j.imu.2023.101332. - DOI
-
- Li G., Li T., Fu W., Hu S. Chapter 16—EGFR- and VEGF(R)-targeted small molecules show synergistic activity in colorectal cancer models refractory to combinations of monoclonal antibodies. In: Hu S., editor. Novel Sensitizing Agents for Therapeutic Anti-EGFR Antibodies. Academic Press; Cambridge, MA, USA: 2023. pp. 119–123.
-
- Cha M.Y., Lee K., Kim M., Song J.Y., Lee K.H., Park J., Chae Y.J., Kim Y.H., Suh K.H., Lee G.S. Antitumor activity of HM781-36B, a highly effective pan-HER inhibitor in erlotinib-resistant NSCLC and other EGFR-dependent cancer models. Int. J. Cancer. 2012;130:2445–2454. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

