Comparison Study of the Safety Profile of Olaparib Versus Niraparib: Analysis of Real-World Data from EudraVigilance
- PMID: 40283963
- PMCID: PMC12030022
- DOI: 10.3390/ph18040528
Comparison Study of the Safety Profile of Olaparib Versus Niraparib: Analysis of Real-World Data from EudraVigilance
Abstract
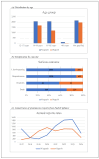
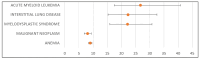
Background: Olaparib and niraparib are poly (ADP-ribose) polymerase inhibitors (PARPi) used primarily for the treatment of ovarian cancer. While both drugs have demonstrated efficacy in clinical trials, their safety profiles, particularly in real-world clinical settings, remain to be fully elucidated. Objectives: This study aimed to (i) characterize the adverse drug reactions (ADRs) associated with olaparib and niraparib as reported in the EudraVigilance database, (ii) compare the frequency of the ADRs occurring during treatment with the two drugs, and (iii) compare post-marketing safety data with those from clinical trials. Methods: A retrospective analysis was performed using data from the EudraVigilance database (2017-2024), focusing on individual case safety reports (ICSRs) related to olaparib and niraparib. Descriptive statistics and disproportionality analysis were performed to compare the frequency and severity of reported ADRs. Results: Both olaparib and niraparib had common ADRs including nausea, vomiting, anemia, thrombocytopenia, and fatigue. However, olaparib was associated with a higher risk of myelodysplastic syndrome (MDS), acute myeloid leukemia (AML), and interstitial lung disease, while niraparib had a higher incidence of gastrointestinal events and thrombocytopenia. Our analysis demonstrates that some specific ADRs, including peripheral neuropathy with niraparib, were reported at higher frequencies compared to clinical trials. The incidence of serious ADRs, including hospitalizations and life-threatening events, was higher with niraparib than with olaparib. Conclusions: This study highlights significant differences in the safety profiles of olaparib and niraparib, with implications for clinical decision-making. Continuous monitoring and personalized management of ADRs are essential to optimize patient outcomes.
Keywords: EudraVigilance; PARP inhibitors; niraparib; olaparib; ovarian cancer; pharmacovigilance; real-world data; safety profile.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Pujade-Lauraine E., Ledermann J.A., Selle F., Gebski V., Penson R.T., Oza A.M., Korach J., Huzarski T., Poveda A., Pignata S., et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–1284. doi: 10.1016/S1470-2045(17)30469-2. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

