Nicotinamide Mononucleotide (NMN) Improves the Senescence of Mouse Vascular Smooth Muscle Cells Induced by Ang II Through Activating p-AMPK/KLF4 Pathway
- PMID: 40283988
- PMCID: PMC12030317
- DOI: 10.3390/ph18040553
Nicotinamide Mononucleotide (NMN) Improves the Senescence of Mouse Vascular Smooth Muscle Cells Induced by Ang II Through Activating p-AMPK/KLF4 Pathway
Abstract
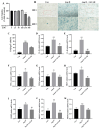
Background: Vascular smooth muscle cells (VSMCs) senescence exacerbates vascular diseases like atherosclerosis and hypertension. Angiotensin II (Ang II) is a strong inducer of VSMCs senescence, causing vascular damage, though its exact mechanism is unclear. Nicotinamide mononucleotide (NMN), a NAD+ precursor, has gained attention for its anti-senescence potential, yet its role in inhibiting VSMCs senescence is not fully understood. Methods: This study assessed senescence markers, including β-galactosidase activity (SA-β-gal) and the senescence-associated secretory phenotype (SASP), in mouse VSMCs treated with Ang II alone or with NMN and relevant activators/inhibitors. Results: Compared to controls, SA-β-gal levels and SASP secretion significantly increased in Ang II-exposed cells. In contrast, NMN reduced the expression of both markers. NMN also reversed Ang II-induced VSMCs senescence by downregulating KLF4 and p16 through AMPK activation, which Ang II inhibited, while decreasing mRNA levels of key SASP components. The effects of the AMPK activator AICAR were similar to those of NMN, whereas the AMPK inhibitor Compound C negated NMN's effects. Conclusions: In summary, NMN mitigates Ang II-induced mouse VSMCs senescence via the AMPK/KLF4/p16 pathway. This study underscores the anti-senescence effects of NMN on mouse VSMCs, supporting further exploration of its potential as a food supplement for preventing and treating vascular senescence.
Keywords: AMPK/KLF4/p16 pathway; Angiotensin II; nicotinamide mononucleotide; senescence; vascular smooth muscle cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
KLF4 prevented angiotensin II-induced smooth muscle cell senescence by enhancing autophagic activity.Eur J Clin Invest. 2022 Sep;52(9):e13804. doi: 10.1111/eci.13804. Epub 2022 May 12. Eur J Clin Invest. 2022. PMID: 35506324
-
α7 Nicotinic Acetylcholine Receptor Relieves Angiotensin II-Induced Senescence in Vascular Smooth Muscle Cells by Raising Nicotinamide Adenine Dinucleotide-Dependent SIRT1 Activity.Arterioscler Thromb Vasc Biol. 2016 Aug;36(8):1566-76. doi: 10.1161/ATVBAHA.116.307157. Epub 2016 Jun 23. Arterioscler Thromb Vasc Biol. 2016. PMID: 27339462
-
Ciprofloxacin Accelerates Angiotensin-II-Induced Vascular Smooth Muscle Cells Senescence Through Modulating AMPK/ROS pathway in Aortic Aneurysm and Dissection.Cardiovasc Toxicol. 2024 Sep;24(9):889-903. doi: 10.1007/s12012-024-09892-z. Epub 2024 Aug 13. Cardiovasc Toxicol. 2024. PMID: 39138741 Free PMC article.
-
Nicotinamide Mononucleotide (NMN) Ameliorates Free Fatty Acid-Induced Pancreatic β-Cell Dysfunction via the NAD+/AMPK/SIRT1/HIF-1α Pathway.Int J Mol Sci. 2024 Sep 30;25(19):10534. doi: 10.3390/ijms251910534. Int J Mol Sci. 2024. PMID: 39408861 Free PMC article.
-
Exendin-4 alleviates angiotensin II-induced senescence in vascular smooth muscle cells by inhibiting Rac1 activation via a cAMP/PKA-dependent pathway.Am J Physiol Cell Physiol. 2014 Dec 15;307(12):C1130-41. doi: 10.1152/ajpcell.00151.2014. Epub 2014 Oct 8. Am J Physiol Cell Physiol. 2014. PMID: 25298426
Cited by
-
Rhein alleviates hepatic steatosis in NAFLD mice by activating the AMPK/ACC/SREBP1 pathway to enhance lipid metabolism.Mol Med. 2025 Jul 10;31(1):255. doi: 10.1186/s10020-025-01304-4. Mol Med. 2025. PMID: 40640749 Free PMC article.
References
-
- Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., et al. Heart Disease and Stroke Statistics-2018 Update: A Report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/cir.0000000000000558. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

