From In Vivo Predictive Dissolution to Virtual Bioequivalence: A GastroPlus®-Driven Framework for Generic Candesartan Cilexetil Tablets
- PMID: 40283997
- PMCID: PMC12030460
- DOI: 10.3390/ph18040562
From In Vivo Predictive Dissolution to Virtual Bioequivalence: A GastroPlus®-Driven Framework for Generic Candesartan Cilexetil Tablets
Abstract
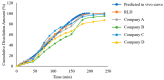
Background: Candesartan cilexetil, a Biopharmaceutics Classification System (BCS) II prodrug, demonstrates compromised bioavailability attributable to its limited aqueous solubility coupled with P-glycoprotein (P-gp)-mediated efflux and hepatic first-pass metabolism, thereby introducing complexities in generic drug bioequivalence assessments. With the rapid advancement of computational technologies, the integration of biorelevant dissolution methodologies with physiologically based pharmacokinetic (PBPK) modeling is emerging as a transformative paradigm in advancing bioequivalence evaluation strategies for generic drug products. This study presents a GastroPlus®-driven framework integrating in vivo predictive dissolution (IPD) and virtual bioequivalence (VBE) to evaluate the quality consistency of generic candesartan cilexetil tablets. Methods: By developing an oral PBPK model in GastroPlus®, we established an IPD method using a phosphate-buffer-based flow-through cell dissolution apparatus. In vitro dissolution profiles of generic tablets from four manufacturers were measured and incorporated into the model to perform VBE simulations. Results: The results demonstrated that only the product from Company A achieved virtual bioequivalence with the reference product, aligning with real-world quality consistency assessments. Conclusions: The proposed framework exhibited robust predictive capability, bridging in vitro dissolution data to in vivo bioequivalence outcomes, thereby offering a cost-effective and efficient strategy for formulation optimization and preclinical bioequivalence evaluation of generic drugs.
Keywords: GastroPlus®; candesartan cilexetil; flow-through cell; in vivo predictive dissolution; virtual bioequivalence.
Conflict of interest statement
Authors Tianjian Ye and Xin Chen were employed by the company Zhejiang Yongning Pharmaceutical Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









Similar articles
-
Establishing virtual bioequivalence and clinically relevant specifications using in vitro biorelevant dissolution testing and physiologically-based population pharmacokinetic modeling. case example: Naproxen.Eur J Pharm Sci. 2020 Feb 15;143:105170. doi: 10.1016/j.ejps.2019.105170. Epub 2019 Nov 27. Eur J Pharm Sci. 2020. PMID: 31783158
-
Utility of Physiologically Based Biopharmaceutics Modeling (PBBM) in Regulatory Perspective: Application to Supersede f2, Enabling Biowaivers & Creation of Dissolution Safe Space.J Pharm Sci. 2022 Dec;111(12):3397-3410. doi: 10.1016/j.xphs.2022.09.003. Epub 2022 Sep 9. J Pharm Sci. 2022. PMID: 36096285
-
Candesartan Cilexetil In Vitro-In Vivo Correlation: Predictive Dissolution as a Development Tool.Pharmaceutics. 2020 Jul 6;12(7):633. doi: 10.3390/pharmaceutics12070633. Pharmaceutics. 2020. PMID: 32640620 Free PMC article.
-
Advancing Virtual Bioequivalence for Orally Administered Drug Products: Methodology, Real-World Applications and Future Outlook.Pharmaceuticals (Basel). 2024 Jul 3;17(7):876. doi: 10.3390/ph17070876. Pharmaceuticals (Basel). 2024. PMID: 39065727 Free PMC article. Review.
-
Physiologically Based Pharmacokinetics Modeling in Biopharmaceutics: Case Studies for Establishing the Bioequivalence Safe Space for Innovator and Generic Drugs.Pharm Res. 2023 Feb;40(2):337-357. doi: 10.1007/s11095-022-03319-6. Epub 2022 Jul 15. Pharm Res. 2023. PMID: 35840856 Review.
References
-
- Liu W., Tu L.X., Yang S.L., Jin Y. Research progress of in vitro and in vivo correlation evaluation method for generic oral solid preparations. Drug Eval. Res. 2020;43:2565–2570.
-
- Mondal P., Roy S., Loganathan G., Mandal B., Dharumadurai D., Akbarsha M.A., Sengupta P.S., Chattopadhyay S., Guin P.S. 1-Amino-4-Hydroxy-9,10-Anthraquinone—An Analogue of Anthracycline Anticancer Drugs, Interacts with DNA and Induces Apoptosis in Human MDA-MB-231 Breast Adinocarcinoma Cells: Evaluation of Structure–Activity Relationship Using Computational, Spectroscopic and Biochemical Studies. Biochem. Biophys. Rep. 2015;4:312–323. doi: 10.1016/j.bbrep.2015.10.008. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

