Tauroursodeoxycholic Acid Protects Retinal Ganglion Cells and Reduces Inflammation in Mice Following Optic Nerve Crush
- PMID: 40284004
- PMCID: PMC12030659
- DOI: 10.3390/ph18040569
Tauroursodeoxycholic Acid Protects Retinal Ganglion Cells and Reduces Inflammation in Mice Following Optic Nerve Crush
Abstract
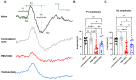
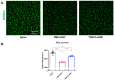
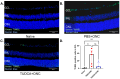
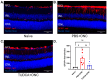
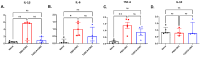
Purpose: The aim of this study was to investigate the protective effects of systemically administered tauroursodeoxycholic acid (TUDCA) in an optic nerve crush (ONC) mouse model of retinal ganglion cell (RGC) death. Methods: C57BL/6J mice were injected intraperitoneally (i.p.) three times per week with TUDCA (500 mg/kg) for two weeks, after which unilateral ONC was performed. A control cohort was identically treated with a drug vehicle (phosphate buffered saline; PBS). A separate cohort did not undergo any injections or surgeries (this was termed the "Naïve" group). Pattern electroretinography (PERG) was recorded 3 days after ONC. Retinas were harvested for whole-mount immunofluorescence staining with an antibody against RGC marker Brn3a and imaged by fluorescent confocal microscopy. Apoptotic cells in the ganglion cell layer (GCL) were detected by Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End Labeling (TUNEL) performed on fixed retina sections. Glial fibrillary acidic protein (GFAP) immunostaining on fixed retina sections was conducted to detect the activation of Müller cells. Total RNA was extracted from retinas and expression of interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and IL-10 was determined by digital droplet PCR (ddPCR). Results: TUDCA treatment preserved visual function as assessed by PERG. P1 and N2 amplitudes from the PBS-treated ONC group were significantly diminished compared to those of the Naïve group (p < 0.001). TUDCA treatment prevented this diminution. The amplitudes of P1 and N2 in the TUDCA-treated ONC group were statistically indistinguishable from those of the Naïve group and were higher than the PBS-treated ONC group (TUDCA+ONC vs. PBS+ONC, P1: 6.99 ± 0.89 µV vs. 3.60 ± 0.69 µV, p < 0.01; N2: -9.30 (IQR: -13.43--6.44) µV vs. -4.47 (IQR: -10.26--2.17) µV). TUDCA treatment preserved RGCs. The ONC-vehicle-only group had 25% fewer RGCs (Brn3a-positive cells) than Naïve eyes (p < 0.0001). TUDCA treatment nearly completely prevented this loss, preserving all but 7.7% of the RGCs, and the number of RGCs in the TUDCA-treated ONC group was significantly higher than in the PBS-treated ONC group (TUDCA+ONC vs. PBS+ONC, 1738.00 ± 14.43 cells per field vs. 1454.00 ± 6.55 cells per field, p < 0.0001). The number of TUNEL-positive cells in the GCL (Naïve vs. PBS+ONC group: 1.00 (IQR: 0.00-2.00) % vs. 37.00 (IQR: 8.50-48.50) %, p < 0.05) and GFAP-positive fibers transversing retina sections (Naïve vs. PBS+ONC group: 33.00 ± 1.15 vs. 185.70 ± 42.37 fibers/retina, p < 0.05), and the expression of IL-6, TNF-α were significantly greater in the PBS-treated ONC group compared to that of the Naïve group (Naïve vs. PBS+ONC group, IL-6: 0.07 (IQR: 0.06-0.31) vs. 0.99 (IQR: 0.56-1.47), p < 0.05, TNF-α: 0.19 ± 0.069 vs. 1.39 ± 0.23; p < 0.01), an increase not observed with TUDCA treatment. Conclusions: Systemic TUDCA treatment significantly preserved RGC function and survival in the mouse ONC model of RGC damage. TUDCA treatment prevented RGC apoptosis, Müller glial cell activation, and retinal expression of several inflammatory cytokines. These data suggest that TUDCA is a promising therapeutic candidate for preserving RGC numbers and function.
Keywords: bile acid; inflammation; mice; mouse; mus musculus; optic nerve crush; retinal ganglion cell; tauroursodeoxycholic acid.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Gupta D., Chen P.P. Glaucoma. Am. Fam. Physician. 2016;93:668–674. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

