Red Blood Cell-Based Delivery Systems for the Release of Hemoglobin-Derived Peptides with In Vitro Antitumor Activities
- PMID: 40284005
- PMCID: PMC12030676
- DOI: 10.3390/ph18040570
Red Blood Cell-Based Delivery Systems for the Release of Hemoglobin-Derived Peptides with In Vitro Antitumor Activities
Abstract
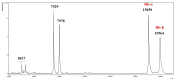
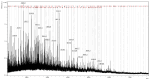
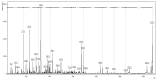
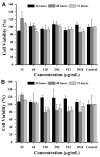
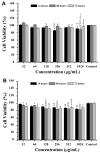
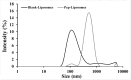
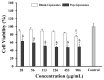
Background/Objectives: This study aimed to develop liposomes derived from lipids obtained from red blood cell membranes for potential use in antitumor applications. Hemoglobin hydrolysates exhibiting peptides with known antitumor activities were encapsulated within these liposomes. Methods: The developed liposomal systems were characterized by their physicochemical properties, including size, surface charge, and encapsulation efficiency, and tested in vitro against 4T1 breast cancer cells and NIH3T3 fibroblasts. Results: Results indicated that the liposomes achieved effective encapsulation (88.9%), with nanometer-scale sizes (ranging from 140.7 nm for Blank-Liposomes to 658.3 nm for Pep-Liposomes) and stable colloidal properties. Conclusions: Although cytotoxicity was limited, the use of liposomes from endogenous components, such as red blood cells, demonstrates promise as a complementary approach in anticancer therapy.
Keywords: antitumor; cell mimetics; in vitro; liposome; mammalian cell; nanosystems; peptides; red blood cell.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Sah A.K., Ramchade A., Ram A., Jain S.K. Resealed erythrocytes: A novel carrier for drug targeting. J. Chem. Pharm. Res. 2011;3:550–565.
-
- Shah S. Novel drug delivery carrier: Resealed erythrocytes. Int. J. Pharma Bio Sci. 2011;2:394–406.
Grants and funding
- Finance Code 001 and no. 23038.019088/2009-58/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
- 311825/2021-4, 307853/2018-7, 408857/2016-1, 306413/2014-0, and 563802/2010-3/Conselho Nacional de Desenvolvimento Científico e Tecnológico
- 193.000.445/2008 and 193.000.429/2008/Fundação de Apoio à Pesquisa do Distrito Federal
- 10.20.03.009.00.00, 23.17.00.069.00.02, 13.17.00.037.00.00, 21.14.03.001.03.05, 13.14.03.010.00.02, 12.16.04.010.00.06, 22.16.05.016.00.04, 11.13.06.001.06.03, and 10.19.03.054.00/Empresa Brasileira de Pesquisa Agropecuária
LinkOut - more resources
Full Text Sources
Miscellaneous

