Recent Developments of 1,3,4-Thiadiazole Compounds as Anticancer Agents
- PMID: 40284015
- PMCID: PMC12030488
- DOI: 10.3390/ph18040580
Recent Developments of 1,3,4-Thiadiazole Compounds as Anticancer Agents
Abstract
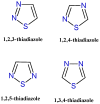
The World Health Organization has recently underlined the increasing global burden of cancer, with a particularly alarming impact on underserved populations. In recent years, 1,3,4-thiadiazole has emerged as a versatile pharmacophore to obtain bioactive compounds. The pharmacological properties of this ring are primarily attributed to its role as a bioisostere of pyrimidine, the core structure of three nucleic bases. This structural feature endows 1,3,4-thiadiazole derivatives with the ability to interfere with DNA replication processes. Additionally, the mesoionic behavior of this heterocycle gives it important properties, such as the ability to cross biological membranes and interact with target proteins. Noteworthy, in analogy to the other sulfur heterocycles, the presence of C-S σ* orbitals, conferring small regions of low electron density on the sulfur atom, makes interaction with the target easier. This review focuses on the most promising anticancer agents with 1,3,4-thiadiazole structure reported in the past five years, providing information that may be useful to medicinal chemists who intend to develop new anticancer derivatives.
Keywords: 1,3,4-thiadiazoles; anticancer agents; breast cancer; pancreatic ductal adenocarcinoma; sulfur-containing heterocycles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Thiadiazole-a promising structure in medicinal chemistry.ChemMedChem. 2013 Jan;8(1):27-41. doi: 10.1002/cmdc.201200355. Epub 2012 Dec 3. ChemMedChem. 2013. PMID: 23208773 Review.
-
1,3,4-thiadiazole: a privileged scaffold for drug design and development.Curr Top Med Chem. 2021;21(28):2546-2573. doi: 10.2174/1568026621666211111154342. Curr Top Med Chem. 2021. PMID: 34766891 Review.
-
Thiadiazole derivatives as anticancer agents.Pharmacol Rep. 2020 Oct;72(5):1079-1100. doi: 10.1007/s43440-020-00154-7. Epub 2020 Sep 3. Pharmacol Rep. 2020. PMID: 32880874 Free PMC article. Review.
-
1,3,4-Thiadiazole: A Versatile Pharmacophore of Medicinal Significance.Med Chem. 2023;19(8):730-756. doi: 10.2174/1573406419666230102104648. Med Chem. 2023. PMID: 36593699 Review.
-
Biological activity of oxadiazole and thiadiazole derivatives.Appl Microbiol Biotechnol. 2022 May;106(9-10):3489-3505. doi: 10.1007/s00253-022-11969-0. Epub 2022 May 14. Appl Microbiol Biotechnol. 2022. PMID: 35562490 Free PMC article. Review.
References
-
- Meanwell N.A. Chapter Five—A Synopsis of the Properties and Applications of Heteroaromatic Rings in Medicinal Chemistry. In: Scriven E.F.V., Ramsden C.A., editors. Advances in Heterocyclic Chemistry. Volume 123. Academic Press; Cambridge, MA, USA: 2017. pp. 245–361.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

