Diversified Soil Types Differentially Regulated the Peanut (Arachis hydropoaea L.) Growth and Rhizosphere Bacterial Community Structure
- PMID: 40284057
- PMCID: PMC12030640
- DOI: 10.3390/plants14081169
Diversified Soil Types Differentially Regulated the Peanut (Arachis hydropoaea L.) Growth and Rhizosphere Bacterial Community Structure
Abstract
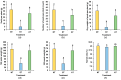
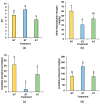
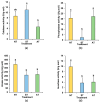
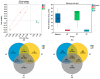
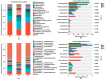
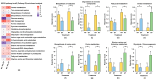
Peanut (Arachis hydropoaea L.) demonstrates a prominent adaptability to diverse soil types. However, the specific effects of soil types on peanut growth and bacterial communities remain elusive. This study conducted a thorough examination of the agronomic traits, the corresponding physicochemical properties, and bacterial structure of rhizosphere soil in acidic (AT), neutral (NT), and saline-alkali (ST) soils, elucidating the internal relationship between soil type and peanut yield. Our results showed that different soil types exhibited significant differences in peanut yield, with ST demonstrating the lowest yield per plant, showing an 85.05% reduction compared to NT. Furthermore, available phosphorus content, urease, and invertase activities were substantially reduced in both ST and AT, particularly in ST by 95.35%, 38.57%, and 62.54%, respectively. Meanwhile, metagenomic sequencing unveiled a notable decline in Bradyrhizobium and Streptomyces in these soils, which is crucial for soil improvement. Further metabolic pathway analysis revealed that the reduction in pathways related to soil remediation, fertility improvement, and stress response in AT and ST may lead to slower peanut growth. In conclusion, peanuts cultivated in acidic and saline-alkali soils can increase yield via implementing soil management practices such as improving soil quality and refining micro-environments. Our study provides practical applications for enhancing peanut yield in low- to medium-yield fields.
Keywords: acidic soil; metagenome; peanut (Arachis hydropoaea L.); rhizosphere bacterial community; saline–alkali soil; soil type.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
The synergy effect of arbuscular mycorrhizal fungi symbiosis and exogenous calcium on bacterial community composition and growth performance of peanut (Arachis hypogaea L.) in saline alkali soil.J Microbiol. 2021 Jan;59(1):51-63. doi: 10.1007/s12275-021-0317-3. Epub 2020 Nov 17. J Microbiol. 2021. PMID: 33201434
-
Arbuscular Mycorrhizal Fungi Restored the Saline-Alkali Soil and Promoted the Growth of Peanut Roots.Plants (Basel). 2023 Sep 28;12(19):3426. doi: 10.3390/plants12193426. Plants (Basel). 2023. PMID: 37836166 Free PMC article.
-
Effects of microbial agent and microbial fertilizer input on soil microbial community structure and diversity in a peanut continuous cropping system.J Adv Res. 2024 Oct;64:1-13. doi: 10.1016/j.jare.2023.11.028. Epub 2023 Nov 28. J Adv Res. 2024. PMID: 38030126 Free PMC article.
-
Comprehensive effects of salt stress and peanut cultivars on the rhizosphere bacterial community diversity of peanut.Arch Microbiol. 2021 Dec 11;204(1):15. doi: 10.1007/s00203-021-02619-6. Arch Microbiol. 2021. PMID: 34894277
-
Short-term continuous monocropping reduces peanut yield mainly via altering soil enzyme activity and fungal community.Environ Res. 2024 Mar 15;245:117977. doi: 10.1016/j.envres.2023.117977. Epub 2023 Dec 21. Environ Res. 2024. PMID: 38141923
References
-
- Ci D., Tang Z., Ding H., Cui L., Zhang G., Li S., Dai L., Qin F., Zhang Z., Yang J., et al. The synergy effect of arbuscular mycorrhizal fungi symbiosis and exogenous calcium on bacterial community composition and growth performance of peanut (Arachis hypogaea L.) in saline alkali soil. J. Microbiol. 2021;59:51–63. doi: 10.1007/s12275-021-0317-3. - DOI - PubMed
-
- Zhao C.X., Jia L.H., Wang Y.F., Wang M.L., Mcgiffen M.E. Effects of Different Soil Texture on Peanut Growth and Development. Commun. Soil Sci. Plant Anal. 2015;46:2249–2257. doi: 10.1080/00103624.2015.1059845. - DOI
-
- Steiner F., da Queiroz L.F.M., Zuffo A.M., da Silva K.C., Lima I.M.D. Peanut response to co-inoculation of spp. and Azospirillum brasilense and molybdenum application in sandy soil of the Brazilian Cerrado. Agron. J. 2021;113:623–632. doi: 10.1002/agj2.20519. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

