Effect of Elaeagnus angustifolia Linn. on the Physicochemical Properties and Microbial Community Structure of Inter-Rhizosphere Soils
- PMID: 40284129
- PMCID: PMC12030227
- DOI: 10.3390/plants14081242
Effect of Elaeagnus angustifolia Linn. on the Physicochemical Properties and Microbial Community Structure of Inter-Rhizosphere Soils
Abstract
Aims: The aim of this study was to elucidate the effect of Elaeagnus angustifolia Linn. (E. angustifolia L.) on the structure and abundance of the soil microbial community. This paper provides a theoretical foundation for guiding the establishment of E. angustifolia L. forests to enhance the physicochemical properties of soil.
Methods: This study employed high-throughput sequencing technology to analyse the composition, diversity, and structural changes of various soil fungal and bacterial communities and correlated the results with soil physicochemical properties.
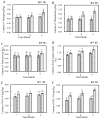
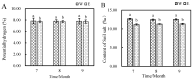
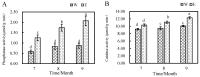
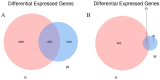
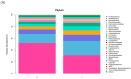
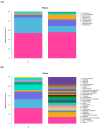
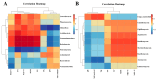
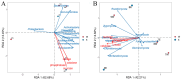
Results: The results indicated a significant increase in the total nitrogen (0.66 g/kg-0.87 g/kg), ammonium nitrogen (3.60 mg/kg-6.56 mg/kg), and organic matter (1.06-1.38%) contents of the inter-rhizosphere soil of E. angustifolia L. after 3, 4, and 5 months of planting. Additionally, the total phosphorus, potassium, and nitrate nitrogen contents increased, whereas soil pH and salinity decreased. The abundance of soil microbial communities also increased. The fungal phyla with relative abundances greater than 1% were Ascomycota, Fungi_unclassified, Basidiomycota, Zygomycota, and Glomeromycota. Chytridiomycota, Rozellomycota, Mortierellomycota, and Olpidiomycota were not found in the bare soil control but were observed in the rhizosphere soil of the date palm. The relative abundance of bacteria from the phyla Proteobacteria, Acidobacteria, Actinobacteria, Gemmatimonadetes, and Chloroflexi in the inter-root soil of jujube dates showed an increase in comparison with the control group. At the same time, correlation analysis found that soil total phosphorus, nitrogen content, and soil enzyme activity were positively correlated with the bacterial level, with TN (p < 0.01) and NO3--N (p < 0.05) showing significant positive correlations. Conversely, soil pH and salinity were mostly negatively correlated with the fungi, and soil enzyme activity was significantly correlated with the fungal and bacterial at different RAD levels.
Conclusions: The introduction of E. angustifolia L. markedly affected the physicochemical properties and microbial community composition of the soil.
Keywords: high-throughput sequencing; inter-rooted soils of E. angustifolia L.; properties of soil in terms of physics and chemistry; soil microbial community structure.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Zhang M., O’Connor P.J., Zhang J., Ye X. Linking soil nutrient cycling and microbial community with vegetation cover in riparian zone. Geoderma. 2021;384:114801. doi: 10.1016/j.geoderma.2020.114801. - DOI
-
- Wang X., Wang X., Wang W., Wang J., Yu F. Effects of invasive plant diversity on soil microbial communities. Diversity. 2022;14:992. doi: 10.3390/d14110992. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

