Endophytes in Cannabis sativa: Identifying and Characterizing Microbes with Beneficial and Detrimental Effects on Plant Health
- PMID: 40284136
- PMCID: PMC12030312
- DOI: 10.3390/plants14081247
Endophytes in Cannabis sativa: Identifying and Characterizing Microbes with Beneficial and Detrimental Effects on Plant Health
Abstract
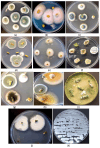
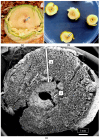
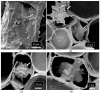
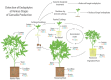
The roles of endophytes in Cannabis sativa (cannabis, hemp) remain poorly explored. While in vitro studies suggest that there can be several benefits, such as plant growth promotion and protection against pathogens, more in planta studies are needed. This review summarizes the bacterial and fungal endophytes previously reported in tissues of C. sativa and discusses the factors influencing their presence, as well as their potential beneficial and detrimental effects. Using genome sequencing and culture-based approaches, we describe the microbial diversity in hydroponically cultivated cannabis plants at several developmental stages. These include mother plants, cuttings, vegetative and flowering plants, and tissue-cultured plantlets. Microbes that were present include fungal, yeast, and bacterial endophytes found in roots, stems, leaves, inflorescences, and seeds. These may have originated from the growing substrate or be transmitted through vegetative propagation. Notable endophytes included Rhizophagus irregularis (a mycorrhizal fungus), Penicillium chrysogenum (an antibiotic producer), and various endophytic yeast species not previously described in C. sativa. Endophytes representing potential plant pathogens, such as Fusarium oxysporum, are also present within cannabis tissues, which can negatively impact plant health. Using scanning electron microscopy, we observed that fungal propagules are present within pith parenchyma cells and xylem vessel elements in stem tissues, illustrating for the first time the in situ localization and distribution of endophytes in cannabis vascular tissues. The mechanism of spread through xylem vessels likely contributes to the spread of endophytes within cannabis and hemp plants. Further research is required to validate the roles of endophytes in cannabis and hemp plants grown under commercial production conditions.
Keywords: biological control; microbial diversity; plant microbiome; plant pathogens; soil microbes.
Conflict of interest statement
Author Liam Buirs is employed by the company Pure Sunfarms Corp. The remaining author (Zamir K. Punja) declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Pure Sunfarms Corp. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Figures















References
-
- Long T., Wagner M., Demske D., Leipe C., Tarasov P.E. Cannabis in Eurasia: Origin of human use and Bronze Age trans-continental connections. Veg. Hist. Archaeobot. 2017;26:245–258. doi: 10.1007/s00334-016-0579-6. - DOI
-
- Small E. Classification of Cannabis sativa L. in relation to agricultural, biotechnological, medical and recreational utilization. In: Chandra S., Lata H., ElSohly M.A., editors. Cannabis sativa L.—Botany and Biotechnology. Springer International Publishing; Cham, Switzerland: 2017. pp. 1–63.
-
- Li H.L. An archaeological and historical account of cannabis in China. Econ. Bot. 1973;28:437–448. doi: 10.1007/BF02862859. - DOI
-
- Fleming M.P., Clarke R.C. Physical evidence for the antiquity of Cannabis sativa L. (Cannabaceae) J. Int. Hemp Assoc. 1998;5:80–92.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

