Faecalibacterium duncaniae Mitigates Intestinal Barrier Damage in Mice Induced by High-Altitude Exposure by Increasing Levels of 2-Ketoglutaric Acid
- PMID: 40284246
- PMCID: PMC12030221
- DOI: 10.3390/nu17081380
Faecalibacterium duncaniae Mitigates Intestinal Barrier Damage in Mice Induced by High-Altitude Exposure by Increasing Levels of 2-Ketoglutaric Acid
Abstract
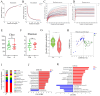
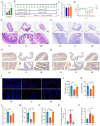
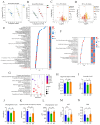
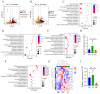
Background/Objectives: Exposure to high altitudes often results in gastrointestinal disorders. This study aimed to identify probiotic strains that can alleviate such disorders. Methods: We conducted a microbiome analysis to investigate the differences in gut microbiota among volunteers during the acute response and acclimatization phases at high altitudes. Subsequently, we established a mouse model of intestinal barrier damage induced by high-altitude exposure to further investigate the roles of probiotic strains and 2-ketoglutaric acid. Additionally, we performed untargeted metabolomics and transcriptomic analyses to elucidate the underlying mechanisms. Results: The microbiome analysis revealed a significant increase in the abundance of Faecalibacterium prausnitzii during the acclimatization phase. Faecalibacterium duncaniae (F. duncaniae) significantly mitigated damage to the intestinal barrier and the reduction of 2-ketoglutaric acid levels in the cecal contents induced by high-altitude exposure in mice. Immunohistochemistry and TUNEL staining demonstrated that high-altitude exposure significantly decreased the expression of ZO-1 and occludin while increasing apoptosis in ileal tissues. In contrast, treatment with F. duncaniae alleviated the loss of ZO-1 and occludin, as well as the apoptosis induced by high-altitude exposure. Furthermore, 2-ketoglutaric acid also mitigated this damage, reducing the loss of occludin and apoptosis in mice. Transcriptomic analysis indicated that high-altitude exposure significantly affects the calcium signaling pathway; conversely, the administration of F. duncaniae significantly influenced the PPAR signaling pathway, mineral absorption, and the regulation of lipolysis in adipocytes. Additionally, the expression of the FBJ osteosarcoma oncogene (Fos) was markedly reduced following the administration of F. duncaniae. Conclusions:F. duncaniae mitigates hypoxia-induced intestinal barrier damage by increasing levels of 2-ketoglutaric acid and shows promise as a probiotic, ultimately aiding travelers in adapting to high-altitude environments.
Keywords: 2-ketoglutaric acid; gastrointestinal issues; gut microbiota; hypoxia exposure; probiotics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Faecalibacterium duncaniae as a novel next generation probiotic against influenza.Front Immunol. 2024 Mar 12;15:1347676. doi: 10.3389/fimmu.2024.1347676. eCollection 2024. Front Immunol. 2024. PMID: 38590519 Free PMC article.
-
Faecalibacterium prausnitzii Supplementation Prevents Intestinal Barrier Injury and Gut Microflora Dysbiosis Induced by Sleep Deprivation.Nutrients. 2024 Apr 9;16(8):1100. doi: 10.3390/nu16081100. Nutrients. 2024. PMID: 38674791 Free PMC article.
-
Resveratrol attenuates non-steroidal anti-inflammatory drug-induced intestinal injury in rats in a high-altitude hypoxic environment by modulating the TLR4/NFκB/IκB pathway and gut microbiota composition.PLoS One. 2024 Aug 12;19(8):e0305233. doi: 10.1371/journal.pone.0305233. eCollection 2024. PLoS One. 2024. PMID: 39133675 Free PMC article.
-
Direct and macrophage stimulation mediated effects of active, inactive, and cell-free supernatant forms of Akkermansia muciniphila and Faecalibacterium duncaniae on hepcidin gene expression in HepG2 cells.Arch Microbiol. 2024 Jun 4;206(7):287. doi: 10.1007/s00203-024-04007-2. Arch Microbiol. 2024. PMID: 38833010
-
Activation of notch signaling pathway is a potential mechanism for mucin2 reduction and intestinal mucosal barrier dysfunction in high-altitude hypoxia.Sci Rep. 2025 Apr 9;15(1):12154. doi: 10.1038/s41598-025-96176-3. Sci Rep. 2025. PMID: 40204779 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

