Polyvinyl Alcohol-Based Membranes: A Review of Research Progress on Design and Predictive Modeling of Properties for Targeted Application
- PMID: 40284281
- PMCID: PMC12030392
- DOI: 10.3390/polym17081016
Polyvinyl Alcohol-Based Membranes: A Review of Research Progress on Design and Predictive Modeling of Properties for Targeted Application
Abstract
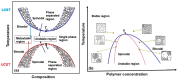
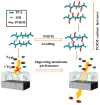
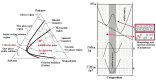
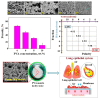
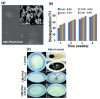
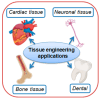
This review provides a comprehensive evaluation of the current state of polyvinyl alcohol (PVA)-based membranes, emphasizing their significance in membrane technology for various applications. The analysis encompasses both experimental and theoretical research articles, with a focus on recent decades, aiming to elucidate the potential and limitations of different fabrication approaches, structure-property relationships, and their applicability in the real world. The review begins by examining the advanced polymeric materials and strategies employed in the design and processing of membranes with tailored properties. Fundamental principles of membrane processes are introduced, with a focus on general modeling approaches for describing the fluid transport through membranes. A key aspect of discussion is the distinction between the membrane performance and process performance. Additionally, an in-depth analysis of PVA membranes in various applications is presented, particularly in environmental fields (e.g., fuel cell, water treatment, air purification, and food packaging) and biomedical domains (e.g., drug delivery systems, wound healing, tissue engineering and regenerative medicine, hemodialysis and artificial organs, and ophthalmic and periodontal treatment). Special attention is given to the relationship between membranes' characteristics, such as material composition, structure, and processing parameters, and their overall performance, in terms of permeability, selectivity, and stability. Despite their promising properties, enhanced through innovative fabrication methods that expand their applicability, challenges remain in optimizing long-term stability, improving fouling resistance, and increasing process scalability. Therefore, further research is needed to develop novel modifications and composite structures that overcome these limitations and enhance the practical implementation of PVA-based membranes. By offering a systematic overview, this review aims to advance the understanding of PVA membrane fabrication, properties, and functionality, providing valuable insights for continued development and optimization in membrane technology.
Keywords: biomedical fields; environmental applications; manufacturing membranes; polyvinyl alcohol; thermodynamic modeling.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures











References
-
- Luis P. Chapter 1—Introduction. In: Luis P., editor. Fundamental Modelling of Membrane Systems. Elsevier; Amsterdam, The Netherlands: 2018. pp. 1–23.
-
- Dautzenberg F.M., Mukherjee M. Process intensification using multifunctional reactors. Chem. Eng. Sci. 2001;56:251–267. doi: 10.1016/S0009-2509(00)00228-1. - DOI
-
- Fried J.R. Basic Principles of Membrane Technology By Marcel Mulder (University of Twente, The Netherlands). Kluwer Academic: Dordrecht. 1996. 564 pp. $255.00. ISBN 0-7823-4247-X. J. Am. Chem. Soc. 1997;119:8582. doi: 10.1021/ja975504k. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

