Recent Advances in Combining Waterborne Acrylic Dispersions with Biopolymers
- PMID: 40284292
- PMCID: PMC12030378
- DOI: 10.3390/polym17081027
Recent Advances in Combining Waterborne Acrylic Dispersions with Biopolymers
Abstract
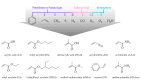
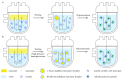
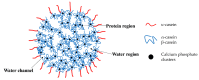
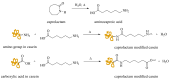
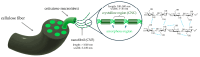
Water-based (meth)acrylic (co)polymer dispersions are produced on a large scale for various applications including coatings, adhesives, paints, and construction materials. A major benefit of waterborne polymer dispersions as compared to more traditional solvent-based alternatives is the low volatile organic compound (VOC) content, which results in an improved environmental profile. Following the trend of sustainability that has driven the growth of acrylic dispersions, recent research has focused on further enhancing the properties of these products by incorporating biobased materials such as polysaccharides (e.g., cellulose, starch, chitin, and chitosan), and proteins (e.g., casein, soy protein, and collagen). Amongst a large number of benefits, the incorporation of biomaterials can serve to decrease the amount of petroleum-based polymers in the formulation and can also contribute to enhance the physical properties of the resulting bio-composites. In this review, the beneficial role of these biopolymers when combined with waterborne acrylic systems is summarized. Recent advances in the use of these biobased and biodegradable materials are covered, aiming to provide guidance for the development of more sustainable, high-performance latex-based bio-composites with minimal environmental impact.
Keywords: biopolymers; casein; cellulose; chitin; chitosan; collagen; nanocellulose; soy protein; starch; waterborne acrylic latexes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Penzel E., Ballard N., Asua J.M. Ullmann’s Encyclopedia of Industrial Chemistry. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2018. Polyacrylates; pp. 1–20.
-
- Topçuoğlu Ö., Altinkaya S.A., Balköse D. Characterization of Waterborne Acrylic Based Paint Films and Measurement of Their Water Vapor Permeabilities. Prog. Org. Coat. 2006;56:269–278. doi: 10.1016/j.porgcoat.2006.02.003. - DOI
-
- Urban D., Egan L. Polymer Dispersions and Their Industrial Applications. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2002. Applications in the Adhesives and Construction Industries; pp. 191–252.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

