Electrospun Chitosan-Coated Recycled PET Scaffolds for Biomedical Applications: Short-Term Antimicrobial Efficacy and In Vivo Evaluation
- PMID: 40284342
- PMCID: PMC12030065
- DOI: 10.3390/polym17081077
Electrospun Chitosan-Coated Recycled PET Scaffolds for Biomedical Applications: Short-Term Antimicrobial Efficacy and In Vivo Evaluation
Abstract
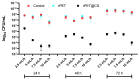
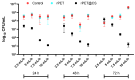
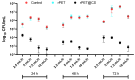
This study investigates the preparation of electrospun recycled polyethylene terephthalate (rPET) coated with chitosan (CS) and evaluates their antibiofilm properties and in vivo response. rPET scaffolds were first fabricated via electrospinning at different flow rates (10, 7.5, 5 and 2.5 mL/h) and subsequently coated with chitosan. Scanning electron microscopy (SEM) revealed that fiber morphology varied with electrospinning parameters, influencing microbial adhesion. Antimicrobial tests demonstrated that rPET@CS significantly inhibited Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans biofilm formation compared to control and uncoated rPET surfaces. Subcutaneous implantation of rPET@CS scaffolds induced a transient inflammatory response, with macrophage recruitment and collagen deposition supporting tissue integration. These findings highlight the potential of rPET@CS scaffolds as sustainable antimicrobial biomaterials for applications in infection-resistant coatings and biomedical implants.
Keywords: Candida albicans; Pseudomonas aeruginosa; Staphylococcus aureus; antimicrobial biomaterials; biofilm inhibition; chitosan; electrospinning; rPET; scaffold; tissue integration.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Mong G.R., Tan H., Chin Vui Sheng D.D., Kek H.Y., Nyakuma B.B., Woon K.S., Othman M.H.D., Kang H.S., Goh P.S., Wong K.Y. A review on plastic waste valorisation to advanced materials: Solutions and technologies to curb plastic waste pollution. J. Clean. Prod. 2024;434:140180. doi: 10.1016/j.jclepro.2023.140180. - DOI
-
- He W., Benson R. Applied Plastics Engineering Handbook. Elsevier; Amsterdam, The Netherlands: 2017. Polymeric biomaterials.
-
- Grumezescu A.M., Stoica A.E., Dima-Bălcescu M.-Ș., Chircov C., Gharbia S., Baltă C., Roșu M., Herman H., Holban A.M., Ficai A., et al. Electrospun Polyethylene Terephthalate Nanofibers Loaded with Silver Nanoparticles: Novel Approach in Anti-Infective Therapy. J. Clin. Med. 2019;8:1039. doi: 10.3390/jcm8071039. - DOI - PMC - PubMed
-
- Al-Sabagh A.M., Yehia F.Z., Eshaq G., Rabie A.M., ElMetwally A.E. Greener routes for recycling of polyethylene terephthalate. Egypt. J. Pet. 2016;25:53–64. doi: 10.1016/j.ejpe.2015.03.001. - DOI
LinkOut - more resources
Full Text Sources

