Biomaterial-Based Additive Manufactured Composite/Scaffolds for Tissue Engineering and Regenerative Medicine: A Comprehensive Review
- PMID: 40284355
- PMCID: PMC12030672
- DOI: 10.3390/polym17081090
Biomaterial-Based Additive Manufactured Composite/Scaffolds for Tissue Engineering and Regenerative Medicine: A Comprehensive Review
Abstract
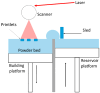
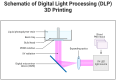
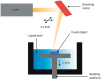
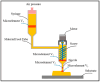
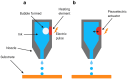
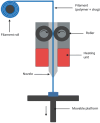
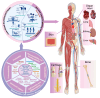
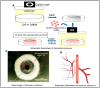
Additive manufacturing (AM), also referred to as three-dimensional printing/printed (3DP), has emerged as a transformative approach in the current design and manufacturing of various biomaterials for the restoration of damaged tissues inside the body. This advancement has greatly aided the development of customized biomedical devices including implants, prosthetics, and orthotics that are specific to the patients. In tissue engineering (TE), AM enables the fabrication of complex structures that promote desirable cellular responses in the regeneration of tissues. Since the choice of biomaterials plays a vital role in scaffold performance as well as cellular responses, meticulous material selection is essential in optimizing the functionality of scaffolds. These scaffolds often possess certain characteristics such as biodegradability, biocompatibility, biomimicry, and porous structure. To this end, polymers such as chitosan, collagen, alginate, hyaluronic acid, polyglycolic acid, polylactic acid, and polycaprolactone have been extensively investigated in the fabrication of tissue-engineered scaffolds. Furthermore, combinations of biomaterials are also utilized to further enhance the scaffolds' performance and functionality. This review discusses the principle of AM and explores recent advancements in AM technologies in the development of TE and regenerative medicine. In addition, the applications of 3DP, polymer-based scaffolds will be highlighted.
Keywords: 3D printing; additive manufacturing; biomaterials; regenerative medicine; scaffold; tissue engineering.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications.Int J Biol Macromol. 2022 Oct 1;218:930-968. doi: 10.1016/j.ijbiomac.2022.07.140. Epub 2022 Jul 24. Int J Biol Macromol. 2022. PMID: 35896130 Review.
-
Three-dimensional (3D) printed scaffold and material selection for bone repair.Acta Biomater. 2019 Jan 15;84:16-33. doi: 10.1016/j.actbio.2018.11.039. Epub 2018 Nov 24. Acta Biomater. 2019. PMID: 30481607 Review.
-
Polymer-Based Scaffolds as an Implantable Material in Regenerative Dentistry: A Review.J Funct Biomater. 2025 Feb 24;16(3):80. doi: 10.3390/jfb16030080. J Funct Biomater. 2025. PMID: 40137359 Free PMC article. Review.
-
3D printed PLA-based scaffolds: a versatile tool in regenerative medicine.Organogenesis. 2013 Oct 1;9(4):239-44. doi: 10.4161/org.26048. Epub 2013 Aug 19. Organogenesis. 2013. PMID: 23959206 Free PMC article.
-
Nanotopographical Features of Polymeric Nanocomposite Scaffolds for Tissue Engineering and Regenerative Medicine: A Review.Biomimetics (Basel). 2025 May 15;10(5):317. doi: 10.3390/biomimetics10050317. Biomimetics (Basel). 2025. PMID: 40422147 Free PMC article. Review.
Cited by
-
Sandwich-Layered Structure Nanofiber Conduits Facilitating the Repair of Long-Gap Nerve Defects.ACS Omega. 2025 Jun 5;10(23):24961-24972. doi: 10.1021/acsomega.5c02439. eCollection 2025 Jun 17. ACS Omega. 2025. PMID: 40547633 Free PMC article.
References
-
- Lanza R., Langer R., Vacanti J. In: Principles of Tissue Engineering. 3rd ed. Lanza R., Langer R., Vacanti J., editors. Academic Press; Cambridge, MA, USA: 2007.
-
- Vyas J., Raytthatha N., Singh S., Prajapati B. Handbook of 3D Printing in Pharmaceutics. CRC Press; Boca Raton, FL, USA: 2024. Next-Generation Computational Automation-Based Additive Manufacturing of Pharmaceuticals: An Approach to Fabricate Precise Medicine; pp. 153–175.
Publication types
LinkOut - more resources
Full Text Sources

