Development of Inhalable Bacteriophage Liposomes Against Pseudomonas aeruginosa
- PMID: 40284401
- PMCID: PMC12030023
- DOI: 10.3390/pharmaceutics17040405
Development of Inhalable Bacteriophage Liposomes Against Pseudomonas aeruginosa
Abstract
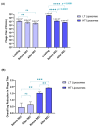
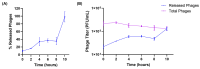
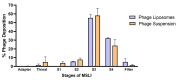
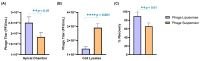
Background:Pseudomonas aeruginosa is one of the major pathogens that cause respiratory infections. The rise of antimicrobial resistance has prompted a need for alternatives to conventional antibiotics. Bacteriophages (phages), natural predators of bacteria, are gaining interest as an alternative therapeutic option against drug-resistant infections. However, phage viability can be lost during manufacturing and delivery. Recent studies show that phages can be taken up by lung epithelial cells, which makes fewer phages available for antibacterial action against extracellular bacteria P. aeruginosa in the airways. Methods: In this study, we encapsulated phages in liposomes using thin film hydration. The effect of processing conditions and phage loading titer on the phage encapsulation and viability was studied. The impact of nebulization on phage viability was tested using an air-jet nebulizer (PARI-LC Plus). Phage cellular uptake was evaluated using an in vitro H441 lung epithelial cell model, grown at the air-liquid interface. Results: Our results demonstrate favorable encapsulation (58 ± 6.02%) can be achieved with minimum loss in phage titer (0.64 ± 0.21 log) by using a low phage titer for hydration. The liposomal formulations exhibited controlled release of phages over 10 h. The formulation also reduced the loss of phage viability during nebulization from 1.55 ± 0.04 log (for phage suspension) to 1.08 ± 0.05 log (for phage liposomes). Encapsulation of phages in liposomes enabled a two-fold reduction in phage cellular uptake and longer extracellular phage retention in the human lung epithelial cell monolayer. Conclusions: Our results indicate that liposomal encapsulation favors phage protection and improves phage availability for antibacterial activity. These findings highlight the potential of liposomes for inhaled phage delivery.
Keywords: bacteria; inhalation; liposomes; nebulization; phages; pulmonary delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Volume 2022. Government of the United Kingdom; London, UK: 2016. p. 10.
-
- Nawaz A., Khalid N.A., Zafar S., Majid A., Shahzadi M., Saleem S., Shah A.A., Badshah M., Khan S. Phage Therapy as a Revolutionary Treatment for Multidrug-Resistant Pseudomonas aeruginosa Infections: A Narrative Review. Microbe. 2024;2:100030. doi: 10.1016/j.microb.2023.100030. - DOI
-
- Chauhan G., Shaik A.A., Sawant S.S., Diwan R., Mokashi M., Goyal M., Shukla S.K., Kunda N.K., Gupta V. Continuously Producible Aztreonam-Loaded Inhalable Lipid Nanoparticles for Cystic Fibrosis-Associated Pseudomonas aeruginosa Infections—Development and in-Vitro Characterization. Biomater. Adv. 2025;166:214027. doi: 10.1016/j.bioadv.2024.214027. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

