Product Development of High-Dose Ambroxol HCl Capsules for an n-of-1 Clinical Trial Involving Dutch Patients with Gaucher Disease Type 3
- PMID: 40284413
- PMCID: PMC12030056
- DOI: 10.3390/pharmaceutics17040417
Product Development of High-Dose Ambroxol HCl Capsules for an n-of-1 Clinical Trial Involving Dutch Patients with Gaucher Disease Type 3
Abstract
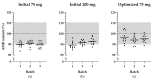
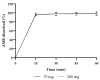
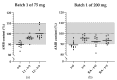
Background/Objectives: Ambroxol hydrochloride (AMB) is a promising chaperone for treating neurological manifestations in Gaucher disease type 3 (GD3). The Amsterdam University Medical Center planned to conduct an n-of-1 clinical trial using high-dose AMB (25 mg/kg/day). As an adequate commercial AMB formulation is unavailable for this high target dosage, we aimed to develop high-dose AMB capsules and assess the formulated capsule's quality. Methods: AMB API was sourced and tested according to the requirements of the European Pharmacopoeia. Capsule formulations of 75 mg and 200 mg AMB were developed. Drug product specifications were set following international guidelines (ICH Q6A) and the European Pharmacopoeia. Analytical methods were developed and validated, and three validation batches of each capsule strength were produced and analyzed. Results: The contents and the Acceptance Values (AVs) of the initial AMB batches (both strengths) varied between 89.1% to 92.7% (specification: 90% to 110%) and 12.4 to 17.6 (specification ≤ 15.0), respectively, indicating non-uniform AMB distribution. Consequently, the production of 200 mg capsules was discontinued, and modifications were made to the 75 mg capsule formulation, followed by the production of three optimized 75 mg validation batches. These batches met the specified criteria, with an AMB content and AV values ranging from 93.9% to 96.5% and 12.4 to 14.9, respectively. Furthermore, rapid dissolution profiles were observed (>80% dissolution within 15 min). No degradation products or microbiological impurities were detected after production. Conclusions: The optimized formulation of 75 mg AMB capsules formulated within the hospital pharmacy setting resulted in qualitative and uniform capsules which can be used in clinical trials.
Keywords: Gaucher disease; active pharmaceutical ingredient (API); ambroxol hydrochloride; drug product specification; good manufacturing practices (GMPs); n-of-1 clinical trial; pharmaceutical quality; product development; product validation; rare diseases.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Charrow J., Andersson H.C., Kaplan P., Kolodny E.H., Mistry P., Pastores G., Rosenbloom B.E., Scott C.R., Wappner R.S., Weinreb N.J., et al. The Gaucher registry: Demographics and disease characteristics of 1698 patients with Gaucher disease. Arch. Intern. Med. 2000;160:2835–2843. doi: 10.1001/archinte.160.18.2835. - DOI - PubMed
-
- Stirnemann J., Belmatoug N., Camou F., Serratrice C., Froissart R., Caillaud C., Levade T., Astudillo L., Serratrice J., Brassier A., et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int. J. Mol. Sci. 2017;18:441. doi: 10.3390/ijms18020441. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

