Verteporfin Inhibits Severe Fever with Thrombocytopenia Syndrome Virus Infection via Inducing the Degradation of the Viral Gn Protein
- PMID: 40284429
- PMCID: PMC12030017
- DOI: 10.3390/pharmaceutics17040434
Verteporfin Inhibits Severe Fever with Thrombocytopenia Syndrome Virus Infection via Inducing the Degradation of the Viral Gn Protein
Abstract
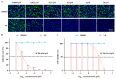
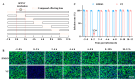
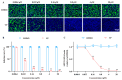
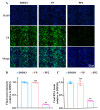
Background: Severe fever with thrombocytopenia syndrome virus (SFTSV) is a novel tick-borne bunyavirus, causing the hemorrhagic infectious disease of SFTS, with a case fatality rate up to 30% due to the absence of effective therapeutic interventions. Therefore, it is urgent to develop safe and effective therapeutic drugs to control this viral hemorrhagic fever. Methods: The activity of verteporfin (VP), screened from an FDA-approved drugs library, against SFTSV, was systematically evaluated in Huh7 cells in a wide range of concentrations. We performed time-of-addition experiments with VP, along with binding, endocytosis, and membrane fusion assays, to determine which part of the SFTSV life cycle VP has its effect on. The potential targets of VP were detected by a drug affinity responsive target stability (DARTS) assay. Results: VP exhibited a potent anti-SFTSV activity by blocking the initial viral binding to the target cells during viral entry via significantly inducing the degradation of the viral Gn protein. Conclusions: The VP-induced inhibition of SFTSV binding, the first step of viral invasion, suggested that VP might be an ideal and potent anti-SFTSV agent due to its prophylaxis and therapeutic effects on viral infection.
Keywords: Gn protein; antiviral agents; severe fever with thrombocytopenia syndrome virus; verteporfin; viral binding.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Antiviral activity and mechanism of the antifungal drug, anidulafungin, suggesting its potential to promote treatment of viral diseases.BMC Med. 2022 Oct 21;20(1):359. doi: 10.1186/s12916-022-02558-z. BMC Med. 2022. PMID: 36266654 Free PMC article.
-
Characterization of Glycoprotein-Mediated Entry of Severe Fever with Thrombocytopenia Syndrome Virus.J Virol. 2016 May 12;90(11):5292-5301. doi: 10.1128/JVI.00110-16. Print 2016 Jun 1. J Virol. 2016. PMID: 26984731 Free PMC article.
-
Interaction between the SFTSV envelope glycoprotein Gn and STING inhibits the formation of the STING-TBK1 complex and suppresses the NF-κB signaling pathway.J Virol. 2024 Mar 19;98(3):e0181523. doi: 10.1128/jvi.01815-23. Epub 2024 Feb 29. J Virol. 2024. PMID: 38421179 Free PMC article.
-
Antiviral Drugs Against Severe Fever With Thrombocytopenia Syndrome Virus Infection.Front Microbiol. 2020 Feb 11;11:150. doi: 10.3389/fmicb.2020.00150. eCollection 2020. Front Microbiol. 2020. PMID: 32117168 Free PMC article. Review.
-
Pathophysiology of severe fever with thrombocytopenia syndrome and development of specific antiviral therapy.J Infect Chemother. 2018 Oct;24(10):773-781. doi: 10.1016/j.jiac.2018.07.009. Epub 2018 Aug 8. J Infect Chemother. 2018. PMID: 30098914 Review.
References
-
- Fang X., Hu J., Peng Z., Dai Q., Liu W., Liang S., Li Z., Zhang N., Bao C. Epidemiological and clinical characteristics of severe fever with thrombocytopenia syndrome bunyavirus human-to-human transmission. PLoS Negl. Trop. Dis. 2021;15:e0009037. doi: 10.1371/journal.pntd.0009037. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

