PEGylation Effects on Amphiphilic Platinum(IV) Complexes: Influence on Uptake, Activation, and Cytotoxicity
- PMID: 40284435
- PMCID: PMC12030465
- DOI: 10.3390/pharmaceutics17040440
PEGylation Effects on Amphiphilic Platinum(IV) Complexes: Influence on Uptake, Activation, and Cytotoxicity
Abstract
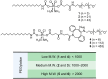
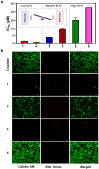
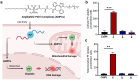
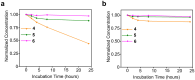
Background/Objectives: The utilization of amphiphilic Pt(IV) complexes as prodrugs offers a promising strategy to revolutionize Pt-based cancer therapy by enhancing drug delivery and activation. While PEGylation is widely used to optimize drug properties, its impact on the biological behavior of amphiphilic Pt(IV) complexes remains unclear. This study systematically investigates how the PEGylation of varying molecular weights influences their cytotoxicity, cellular uptake, and activation. Methods: Pt(IV) complexes were synthesized with PEG chains of different molecular weights using HATU-catalyzed amide bond formation and copper-free click chemistry. Their biological properties were assessed through cell-based analyses, focusing on cytotoxicity, cellular uptake, and activation by biological reductants. Results: Small PEG modifications retained the potent cytotoxicity of amphiphilic Pt(IV) prodrugs, whereas large PEG chains significantly reduced efficacy. The decrease in potency was linked to impaired cellular uptake and mitochondrial accumulation. Additionally, large PEG modifications slowed the reduction and activation of Pt(IV) prodrugs by biological reductants, further limiting their anticancer activities. Conclusions: These findings underscore the critical role of PEGylation in metallodrug design and provide key insights into optimizing PEGylation strategies for enhancing platinum-based cancer therapies.
Keywords: PEGylation; cisplatin; mitochondria; platinum(IV) prodrugs.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Pyrenebutyrate Pt(IV) Complexes with Nanomolar Anticancer Activity.Pharmaceutics. 2023 Sep 13;15(9):2310. doi: 10.3390/pharmaceutics15092310. Pharmaceutics. 2023. PMID: 37765279 Free PMC article.
-
A Ratiometric Fluorescent Probe for Cisplatin: Investigating the Intracellular Reduction of Platinum(IV) Prodrug Complexes.Angew Chem Int Ed Engl. 2019 Jan 2;58(1):164-167. doi: 10.1002/anie.201810361. Epub 2018 Dec 4. Angew Chem Int Ed Engl. 2019. PMID: 30407697
-
Stability, Reduction, and Cytotoxicity of Platinum(IV) Anticancer Prodrugs Bearing Carbamate Axial Ligands: Comparison with Their Carboxylate Analogues.Inorg Chem. 2020 Aug 17;59(16):11676-11687. doi: 10.1021/acs.inorgchem.0c01541. Epub 2020 Aug 3. Inorg Chem. 2020. PMID: 32799457
-
Supramolecular platinum complexes for cancer therapy.Curr Opin Chem Biol. 2023 Apr;73:102276. doi: 10.1016/j.cbpa.2023.102276. Epub 2023 Mar 4. Curr Opin Chem Biol. 2023. PMID: 36878171 Free PMC article. Review.
-
Pt(IV) Complexes in the Search for Novel Platinum Prodrugs with Promising Activity.Top Curr Chem (Cham). 2024 Feb 24;382(1):6. doi: 10.1007/s41061-023-00448-3. Top Curr Chem (Cham). 2024. PMID: 38400859 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

