Umbilical Cord Mesenchymal Stem Cell-Derived Apoptotic Extracellular Vesicles Improve 5-FU-Induced Delayed Wound Healing by Mitochondrial Transfer
- PMID: 40284448
- PMCID: PMC12030720
- DOI: 10.3390/pharmaceutics17040453
Umbilical Cord Mesenchymal Stem Cell-Derived Apoptotic Extracellular Vesicles Improve 5-FU-Induced Delayed Wound Healing by Mitochondrial Transfer
Abstract
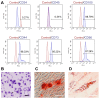
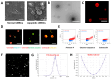
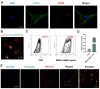
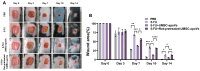
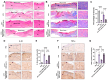
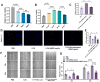
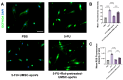
Background/Objectives: This study aimed to explore the therapeutic potential of umbilical mesenchymal stem cell-derived apoptotic vesicles (UMSC-apoVs) in a 5-Fluorouracil (5-FU)-induced impairment in skin wound healing. Methods: UMSC-apoVs were isolated from UMSCs using differential centrifugation after the induction of apoptosis. A murine model was established by administering 5-FU via intravenous tail injection, followed by full-thickness skin wound creation. Mice received local injections of UMSC-apoVs at the lesion site. Wound healing was evaluated based on wound closure rates, histological analysis, and in vivo/in vitro functional assays. Rotenone (Rot)-pretreated UMSC-apoVs were used to explore the role of mitochondrial transfer between skin mesenchymal stem cells (SMSCs) and UMSC-apoVs in wound healing. Results: UMSC-apoVs significantly accelerated wound healing in 5-FU-treated mice, as demonstrated by enhanced wound closure rates and histological findings of reduced inflammatory infiltration and increased collagen deposition. UMSC-apoVs transferred mitochondria to SMSCs, enhancing viability, proliferation, and migration while reducing reactive oxygen species (ROS) production in SMSCs. Rot pretreatment inhibited the therapeutic effects of UMSC-apoVs on wound healing by inducing mitochondrial dysfunction in UMSC-apoVs. Conclusions: Our findings indicate that UMSC-apoVs improve 5-FU-induced impaired skin wound healing by facilitating mitochondrial transfer, suggesting a novel therapeutic strategy for alleviating chemotherapy-induced impairment in wound healing.
Keywords: 5-fluorouracil; delayed wound healing; mitochondrial transfer; skin mesenchymal stem cells; umbilical cord mesenchymal stem cell-derived apoptotic vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Apoptotic vesicles inherit SOX2 from pluripotent stem cells to accelerate wound healing by energizing mesenchymal stem cells.Acta Biomater. 2022 Sep 1;149:258-272. doi: 10.1016/j.actbio.2022.07.009. Epub 2022 Jul 10. Acta Biomater. 2022. PMID: 35830925
-
Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture.Cell Prolif. 2019 Mar;52(2):e12570. doi: 10.1111/cpr.12570. Epub 2019 Jan 20. Cell Prolif. 2019. PMID: 30663158 Free PMC article.
-
Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-β/SMAD2 Pathway During Wound Healing.Stem Cells Transl Med. 2016 Oct;5(10):1425-1439. doi: 10.5966/sctm.2015-0367. Epub 2016 Jul 7. Stem Cells Transl Med. 2016. PMID: 27388239 Free PMC article.
-
Apoptotic vesicles: emerging concepts and research progress in physiology and therapy.Life Med. 2023 Apr 17;2(2):lnad013. doi: 10.1093/lifemedi/lnad013. eCollection 2023 Apr. Life Med. 2023. PMID: 39872110 Free PMC article. Review.
-
Extracellular vesicle-loaded hydrogels for tissue repair and regeneration.Mater Today Bio. 2022 Dec 21;18:100522. doi: 10.1016/j.mtbio.2022.100522. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36593913 Free PMC article. Review.
References
-
- Yuan W., Ji G., Shi X., Sun Z., Liu C., Yu Y., Li W., Wang X., Hu H. The male reproductive toxicity after 5-Fluorouracil exposure: DNA damage, oxidative stress, and mitochondrial dysfunction in vitro and in vivo. Ecotoxicol. Env. Saf. 2024;278:116465. doi: 10.1016/j.ecoenv.2024.116465. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

