Microfluidic Optimization of PEI-Lipid Hybrid Nanoparticles for Efficient DNA Delivery and Transgene Expression
- PMID: 40284449
- PMCID: PMC12030462
- DOI: 10.3390/pharmaceutics17040454
Microfluidic Optimization of PEI-Lipid Hybrid Nanoparticles for Efficient DNA Delivery and Transgene Expression
Abstract
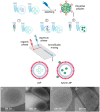
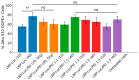
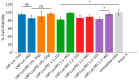
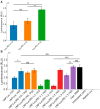
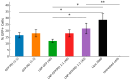
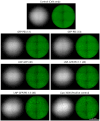
Background: Lipid nanoparticles (LNPs) and polyethyleneimine (PEI) have independently been used for DNA complexation and delivery. However, non-ideal gene delivery efficiency and toxicity have hindered their clinical translation. We developed DNA-PEI-LNPs as a strategy to overcome these limitations and enhance DNA delivery and transgene expression. Methods: Three microfluidic mixing protocols were evaluated: (i) LNPs without PEI, (ii) a single-step process incorporating PEI in the organic phase, and (iii) a two-step process with DNA pre-complexed with PEI before LNP incorporation. The influence of DNA/PEI ratios (1:1, 1:2, 1:3) and DNA/lipid ratios (1:10, 1:40) on particle properties and delivery efficiency was examined. Results: In luciferase formulations, higher DNA/lipid ratios (1:40) produced smaller particles (136 nm vs. 188 nm) with improved cellular uptake (77% vs. 50%). The two-step method with higher DNA/PEI ratios improved transfection efficiency, with LNP-Luc/PEI 1:3 (40) achieving ~1.9 × 106 relative light units (RLU) in luciferase expression. In green fluorescent protein (GFP) studies, LNP-GFP/PEI 1:3 (40) showed ~23.8% GFP-positive cells, nearly twofold higher than LNP-GFP (40) at ~12.6%. Conclusions: These results demonstrate the capability of microfluidic-prepared DNA-PEI-LNPs to improve DNA delivery and transgene expression through optimized formulation strategies and selection of appropriate preparation methods.
Keywords: DNA delivery; GFP expression; gene therapy; lipid nanoparticles (LNPs); lipid-polymer hybrid systems; luciferase assays; microfluidic mixing; polyethyleneimine (PEI); transgene expression.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Dogbey D.M., Torres V.E.S., Fajemisin E., Mpondo L., Ngwenya T., Akinrinmade O.A., Perriman A.W., Barth S. Technological advances in the use of viral and non-viral vectors for delivering genetic and non-genetic cargos for cancer therapy. Drug Deliv. Transl. Res. 2023;13:2719–2738. doi: 10.1007/s13346-023-01362-3. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

