Optimization, In Vitro, and In Silico Characterization of Theophylline Inhalable Powder Using Raffinose-Amino Acid Combination as Fine Co-Spray-Dried Carriers
- PMID: 40284461
- PMCID: PMC12030175
- DOI: 10.3390/pharmaceutics17040466
Optimization, In Vitro, and In Silico Characterization of Theophylline Inhalable Powder Using Raffinose-Amino Acid Combination as Fine Co-Spray-Dried Carriers
Abstract
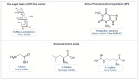
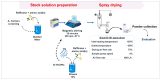
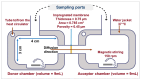
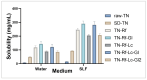
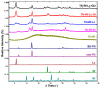
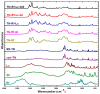
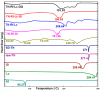
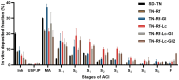
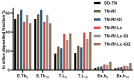
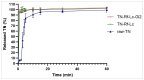
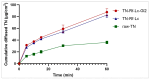
Background/Objectives: Dry powder inhalation is an attractive research area for development. Therefore, this work aimed to develop inhalable co-spray-dried theophylline (TN) microparticles, utilizing raffinose-amino acid fine carriers intended for asthma therapy. The study addressed enhancing TN's physicochemical and aerodynamic properties to ensure efficient lung deposition. Methods: The process involves spray-drying each formulation's solution using a mini spray drier. A rigorous assessment was conducted on particle size distribution, structural and thermal analysis, morphology study, in vitro and in silico aerodynamic investigation, and aerodynamic particle counter in addition to the solubility, in vitro dissolution, and diffusion of TN. Results: The carriers containing leucine and glycine revealed superior characteristics (mass median aerodynamic diameter (MMAD): 4.6-5 µm, fine particle fraction (FPF): 30.6-35.1%, and amorphous spherical structure) as candidates for further development of TN-DPIs, while arginine was excluded due to intensive aggregation and hygroscopicity, which led to poor aerodynamic performance. TN co-spray-dried samples demonstrated fine micronized particles (D [0.5]: 3.99-5.96 µm) with predominantly amorphous structure (crystallinity index: 24.1-45.2%) and significant solubility enhancement (~19-fold). Formulations containing leucine and leucine-glycine revealed the highest FPF (45.7-47.8%) and in silico lung deposition (39.3-40.1%), rapid in vitro drug release (~100% within 10 min), and improved in vitro diffusion (2.29-2.43-fold), respectively. Moreover, the aerodynamic counter confirmed the development of fine microparticles (mean number particle size = 2.3-2.02 µm). Conclusions: This innovative formulation possesses enhanced physicochemical, morphological, and aerodynamic characteristics of low-dose TN for local asthma treatment and could be applied as a promising carrier for dry powder inhaler development.
Keywords: Andersen cascade impactor; aerodynamic particle counter; dry powder inhaler; glycine; leucine; raffinose; spray-drying; stochastic lung model; theophylline.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Enhanced pulmonary delivery of spray-dried theophylline: investigation of the trehalose and amino acid combinations as innovative fine carriers.Eur J Pharm Sci. 2025 Jun 1;209:107109. doi: 10.1016/j.ejps.2025.107109. Epub 2025 Apr 23. Eur J Pharm Sci. 2025. PMID: 40280283
-
Development of extra-fine particles containing nanosized meloxicam for deep pulmonary delivery: In vitro aerodynamic and cell line measurements.Eur J Pharm Sci. 2022 Sep 1;176:106247. doi: 10.1016/j.ejps.2022.106247. Epub 2022 Jun 25. Eur J Pharm Sci. 2022. PMID: 35760279
-
Development of drug alone and carrier-based GLP-1 dry powder inhaler formulations.Int J Pharm. 2022 Apr 5;617:121601. doi: 10.1016/j.ijpharm.2022.121601. Epub 2022 Feb 16. Int J Pharm. 2022. PMID: 35181460
-
Physico-Chemical Properties, Aerosolization and Dissolution of Co-Spray Dried Azithromycin Particles with L-Leucine for Inhalation.Pharm Res. 2018 Jan 8;35(2):28. doi: 10.1007/s11095-017-2334-9. Pharm Res. 2018. PMID: 29374368 Free PMC article.
-
Inhalable co-amorphous budesonide-arginine dry powders prepared by spray drying.Int J Pharm. 2019 Jun 30;565:1-8. doi: 10.1016/j.ijpharm.2019.04.036. Epub 2019 Apr 15. Int J Pharm. 2019. PMID: 30999050
Cited by
-
Development of Mannitol-Based Microparticles for Dry Powder Inhalers: Enhancing Pulmonary Delivery of NSAIDs.Pharmaceuticals (Basel). 2025 Jun 19;18(6):923. doi: 10.3390/ph18060923. Pharmaceuticals (Basel). 2025. PMID: 40573321 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

