Nanostructured Lipid Carriers (NLC)-Based Topical Formulation of Hesperidin for Effective Treatment of Psoriasis
- PMID: 40284473
- PMCID: PMC12030045
- DOI: 10.3390/pharmaceutics17040478
Nanostructured Lipid Carriers (NLC)-Based Topical Formulation of Hesperidin for Effective Treatment of Psoriasis
Abstract
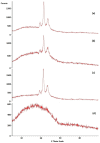
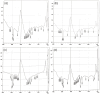
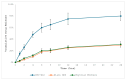
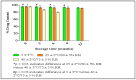
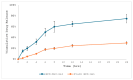
Background: Various routes of drug administration are available for psoriasis treatment. However, there is an urgent need for novel and improved therapeutic options. Hence, our study aimed to develop a nanostructured lipid carrier (NLC) gel of hesperidin (HPD) using a systemic QbD approach for an effective treatment of psoriasis. Methods: Initially, HPD-NLC was optimized with independent variables (drug content, amount of liquid lipid, total lipid, and surfactant concentration) using Box-Behnken Design to assess dependent variables (particle size, size distribution, and entrapment efficiency). HPD-NLC was developed using the high-shear homogenization technique. The characteristics of nanoformulation such as particle size, morphology [transmission electron microscopy (TEM) and differential scanning calorimetry (DSC)], crystallinity [powder X-ray diffraction (XRD)], and chemical interactions [Fourier transform infrared spectroscopy (FTIR)], the drug entrapment efficiency (%EE), and the drug release were investigated. Franz-diffusion cell was utilized to perform in vitro diffusion study, and an imiquimod-induced psoriasis model was used for in vivo study. Results: The optimized HPD-NLC exhibited a spherical shape with particle size of 125.7 nm, polydispersity index (PDI) of 0.36, and entrapment efficiency of 52.26% w/w. Further, different techniques validated the reduced crystallinity of the hesperidin. The in vitro diffusion study highlighted the sustained and anomalous diffusion of the drug from NLC gel. In the in vivo study, the HPD-NLC-Gel-treated group displayed normal skin with minimal keratosis, while the drug-loaded gel group exhibited signs of hyperkeratosis and parakeratosis signs. Conclusions: HPD-NLC gel showed promising advancement in nanotechnology-based psoriasis treatment and the results of this study open the door for the application of topical HPD-NLC-Gel clinically.
Keywords: Box–Behnken Design; hesperidin; high-shear homogenization; nanostructured lipid carriers; psoriasis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Almenara-Blasco M., Gracia-Cazaña T., Poblador-Plou B., Laguna-Berna C., Carmona-Pírez J., Navarro-Bielsa A., Prados-Torres A., Gimeno-Miguel A., Gilaberte Y. Multimorbidity of Psoriasis: A Large-Scale Population Study of Its Associated Comorbidities. J. Clin. Med. 2024;13:492. doi: 10.3390/jcm13020492. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

