Recent Advances in Vitamin E TPGS-Based Organic Nanocarriers for Enhancing the Oral Bioavailability of Active Compounds: A Systematic Review
- PMID: 40284480
- PMCID: PMC12030195
- DOI: 10.3390/pharmaceutics17040485
Recent Advances in Vitamin E TPGS-Based Organic Nanocarriers for Enhancing the Oral Bioavailability of Active Compounds: A Systematic Review
Abstract
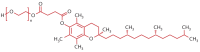
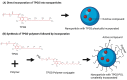
Background: D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS), an amphiphilic derivative of natural vitamin E, functions as both a drug efflux inhibitor and a protector against enzymatic degradation and has been widely incorporated into nano-formulations for drug design and delivery. Objective: This systematic review evaluates TPGS-based organic nanocarriers, emphasizing their potential to enhance bioavailability of active compounds which include drugs and phytochemicals, improve pharmacokinetic profiles, and optimize therapeutic outcomes, eventually overcoming the limitations of conventional oral active compounds delivery. Search strategy: Data collection was carried out by entering key terms (TPGS) AND (Micelle OR Liposome OR Nanoparticle OR Nanotube OR Dendrimer OR Niosome OR Nanosuspension OR Nanomicelle OR Nanocrystal OR Nanosphere OR Nanocapsule) AND (Oral Bioavailability) into the Scopus database. Inclusion criteria: Full-text articles published in English and relevant to TPGS, which featured organic materials, utilized an oral administration route, and included pharmacokinetic study, were included to the final review. Data extraction and analysis: Data selection was conducted by two review authors and subsequently approved by all other authors through a consensus process. The outcomes of the included studies were reviewed and categorized based on the types of nanocarriers. Results: An initial search of the database yielded 173 records. After screening by title and abstract, 52 full-text articles were analyzed. A total of 21 papers were excluded while 31 papers were used in this review. Conclusions: This review concludes that TPGS-based organic nanocarriers are able to enhance the bioavailability of various active compounds, including several phytochemicals, leveraging TPGS's amphiphilic nature, inhibition of efflux transporters, protection against degradation, and stabilization properties. Despite using the same excipient, variability in particle size, zeta potential, and encapsulation efficiency among nanocarriers indicates the need for tailored formulations. A comprehensive approach involving the development and standardized comparison of diverse TPGS-incorporated active compound formulations is essential to identify the optimal TPGS-based nanocarrier for improving a particular active compound's bioavailability.
Keywords: TPGS; drug delivery; oral bioavailability; organic nanocarriers; vitamin E.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
Similar articles
-
Superiority of TPGS-loaded micelles in the brain delivery of vinpocetine via administration of thermosensitive intranasal gel.Int J Nanomedicine. 2019 Jul 23;14:5555-5567. doi: 10.2147/IJN.S213086. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31413562 Free PMC article.
-
Poly(lactide)-vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of Docetaxel.Biomaterials. 2009 Jul;30(19):3297-306. doi: 10.1016/j.biomaterials.2009.02.045. Epub 2009 Mar 19. Biomaterials. 2009. PMID: 19299012
-
Nanocarriers based on vitamin E-TPGS: Design principle and molecular insights into improving the efficacy of anticancer drugs.Int J Pharm. 2021 Jan 5;592:120045. doi: 10.1016/j.ijpharm.2020.120045. Epub 2020 Nov 16. Int J Pharm. 2021. PMID: 33212172 Review.
-
The applications of Vitamin E TPGS in drug delivery.Eur J Pharm Sci. 2013 May 13;49(2):175-86. doi: 10.1016/j.ejps.2013.02.006. Epub 2013 Feb 26. Eur J Pharm Sci. 2013. PMID: 23485439 Review.
-
Vitamin E TPGS as a molecular biomaterial for drug delivery.Biomaterials. 2012 Jun;33(19):4889-906. doi: 10.1016/j.biomaterials.2012.03.046. Epub 2012 Apr 11. Biomaterials. 2012. PMID: 22498300 Review.
Cited by
-
Surfactant-Enabled Nanocarriers in Breast Cancer Therapy: Targeted Delivery and Multidrug Resistance Reversal.Pharmaceutics. 2025 Jun 13;17(6):779. doi: 10.3390/pharmaceutics17060779. Pharmaceutics. 2025. PMID: 40574091 Free PMC article. Review.
-
Advancing cancer therapy: Nanomaterial-based encapsulation strategies for enhanced delivery and efficacy of curcumin.Mater Today Bio. 2025 Jun 9;33:101963. doi: 10.1016/j.mtbio.2025.101963. eCollection 2025 Aug. Mater Today Bio. 2025. PMID: 40575656 Free PMC article. Review.
References
-
- Mir M., Ali A., Ahamad R., Khan Z. Futuristic Trends in Pharmacy & Nursing. IIP Series; Chikkamagaluru, India: 2024. Chemical Stability of Drugs: Unlocking the Future of Pharmacy and Nursing; pp. 59–65.
-
- Khadka P., Ro J., Kim H., Kim I., Kim J.T., Kim H., Cho J.M., Yun G., Lee J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014;9:304–316. doi: 10.1016/j.ajps.2014.05.005. - DOI
-
- Uttreja P., Karnik I., Adel Ali Youssef A., Narala N., Elkanayati R.M., Baisa S., Alshammari N.D., Banda S., Vemula S.K., Repka M.A. Self-Emulsifying Drug Delivery Systems (SEDDS): Transition from Liquid to Solid—A Comprehensive Review of Formulation, Characterization, Applications, and Future Trends. Pharmaceutics. 2025;17:63. doi: 10.3390/pharmaceutics17010063. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

