Three-Dimensional-Printed Bone Grafts for Simultaneous Bone and Cartilage Regeneration: A Promising Approach to Osteochondral Tissue Engineering
- PMID: 40284484
- PMCID: PMC12030389
- DOI: 10.3390/pharmaceutics17040489
Three-Dimensional-Printed Bone Grafts for Simultaneous Bone and Cartilage Regeneration: A Promising Approach to Osteochondral Tissue Engineering
Abstract
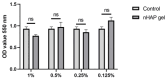
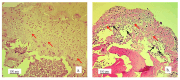
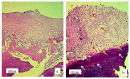
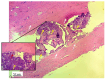
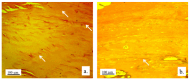
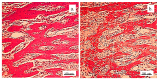
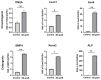
Background/Objectives: A novel 3D-printed, bioresorbable bone graft, made of nanohydroxyapatite (nHAP) covered by poly(lactide-co-glycolide) (PLGA), showed strongly expressed osteoinductive properties in our previous investigations. The current study examines its application in the dual regeneration of bone and cartilage by combining with nHAP gel obtained by nHAP enrichment with hydroxyethyl cellulose, sodium hyaluronate, and chondroitin sulfate. Methods: In the in vitro part of the study, the mitochondrial activity and osteogenic and chondrogenic differentiation of stem cells derived from apical papilla (SCAPs) in the presence of nHAP gel were investigated. For the in vivo part of the study, three rabbits underwent segmental osteotomies of the lateral condyle of the femur, and defects were filled by 3D-printed grafts customized to the defect geometry. Results: In vitro study revealed that nHAP gel displayed significant biocompatibility, substantially increasing mitochondrial activity and facilitating the osteogenic and chondrogenic differentiation of SCAPs. For the in vivo part of the study, after a 12-week healing period, partial resorption of the graft was observed, and lamellar bone tissue with Haversian systems was detected. Histological and stereological evaluations of the implanted grafts indicated successful bone regeneration, marked by the infiltration of new bone and cartilaginous tissue into the graft. The existence of osteocytes and increased vascularization indicated active osteogenesis. The hyaline cartilage near the graft showed numerous new chondrocytes and a significant layer of newly formed cartilage. Conclusions: This study demonstrated that tailored 3D-printed bone grafts could efficiently promote the healing of substantial bone defects and the formation of new cartilage without requiring supplementary biological factors, offering a feasible alternative for clinical bone repair applications.
Keywords: 3D printing; animal model; bone reconstruction; cartilage regeneration; nanohydroxyapatite; segmental osteotomy; stem cells.
Conflict of interest statement
Author Milutin Mićić was employed by the company Otoprint doo and author Vukoman Jokanović was employed by the company ALBOS doo. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










References
-
- Cao X., Wang J., Liu M., Chen Y., Cao Y., Yu X. Chitosan-Collagen/Organomontmorillonite Scaffold for Bone Tissue Engineering. Front. Mater. Sci. 2015;9:405–412. doi: 10.1007/s11706-015-0317-5. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

