The Modulation of Cell Plasticity by Budesonide: Beyond the Metabolic and Anti-Inflammatory Actions of Glucocorticoids
- PMID: 40284499
- PMCID: PMC12030213
- DOI: 10.3390/pharmaceutics17040504
The Modulation of Cell Plasticity by Budesonide: Beyond the Metabolic and Anti-Inflammatory Actions of Glucocorticoids
Abstract
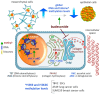
The synthetic cortisol analog budesonide (BUD) is an essential drug employed to manage chronic inflammatory diseases in humans, mainly those involving gastroenteric and airway mucosa, such as rhinitis, laryngitis, bronchitis, esophagitis, gastritis, and colitis, with high levels of success. As a glucocorticoid, BUD prevents the expression of pro-inflammatory cytokines/chemokines and the recruitment of immune cells into the inflamed mucosa. However, emerging evidence indicates that BUD, unlike classical glucocorticoids, is also a potent modulator of stem and cancer cell behavior/plasticity. Certainly, BUD stabilizes cell-cell adhesions, preventing embryonic stem cell differentiation and inhibiting the development of 3D gastruloids. In addition, BUD inhibits the motile/invasive propensity of different cancer cells, including breast, lung, and pancreatic cancer. Finally, it prevents the infection of positive single-stranded human-infecting RNA viruses such as SARS-CoV-2. At a molecular level, BUD induces epigenetic changes and modifies the transcriptome of epithelial, stem, and cancer cells, providing molecular support to the immune cell-independent activity of BUD. Here, we performed an in-depth review of these unexpected activities of BUD, identified by unbiased drug screening programs, and we emphasize the molecular mechanisms modulated by this efficacious drug that deserve further research.
Keywords: SARS-CoV-2; budesonide; cancer cells; gastruloids; metastasis; stem cells.
Conflict of interest statement
The authors have no financial, non-financial, or proprietary interests in any material discussed in this article. The authors are neither employed by nor work as consultants for any company/organization that may gain or lose financially through the publication of this manuscript. The arguments presented in this review are not based on the authors’ professional interests or personal beliefs.
Figures





References
Publication types
Grants and funding
- IG20736/Italian Foundation for Cancer Research (AIRC)
- 2022KME7RY/Italian Ministry of University and Research (MUR-PRIN)
- P20224ZY5P/Italian Ministry of University and Research (MUR-PRIN)
- PNC 0000001 D34Health/Next Generation EU, in the context of the National Plan for Complementary Investments (PNC) to the National Recovery and Resilience Plan (PNRR)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

