Intracellular Protein Binding of Zr-89 Oxine Cell Labeling for PET Cell Tracking Studies
- PMID: 40284513
- PMCID: PMC12030610
- DOI: 10.3390/pharmaceutics17040518
Intracellular Protein Binding of Zr-89 Oxine Cell Labeling for PET Cell Tracking Studies
Abstract
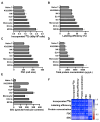
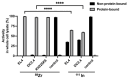
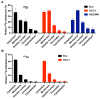
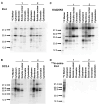
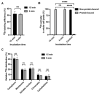
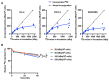
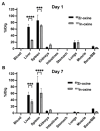
Background/Objectives: 89Zr-oxine is an ex vivo cell labeling agent that enables cells to be tracked in vivo by positron emission tomography (PET) over a period of up to two weeks. To better understand where 89Zr-oxine binds within cellular components, factors affecting labeling and intracellular distribution of 89Zr were examined. Methods: Mouse primary T cells, natural killer cells, dendritic cells, and monocytes, and cell lines EL4 (mouse lymphoma), DC2.4 (mouse dendritic cell), Kit225K6 (human T cell leukemia) and MC38 (mouse colon adenocarcinoma) were labeled with 89Zr-oxine or 111In-oxine and protein binding within the cellular compartments, the labeling thresholds, and radioactivity retention were subsequently determined. Results: Cell incorporation of 89Zr-oxine (27.8-71.8 kBq/106 cells) positively correlated with cellular size and protein mass. Most (>97%) 89Zr was protein-bound and primarily localized in the cytoplasm, membrane, and nuclear fractions (>81%) with distribution patterns varying by cell type. By contrast, 111In-oxine showed lower protein-binding activity of approximately 59-65%, with 62-65% of 111In localized in the cytoplasm. Autoradiography of electrophoresed subcellular fractionated cell samples indicated stable binding by 89Zr-oxine to proteins in all subcellular fractions but unstable protein binding by 111In. Saturation studies showed that 89Zr-oxine labeling was saturable, and further labeling reduced cellular retention. Biodistribution of dendritic cells labeled with either 89Zr-oxine or 111In-oxine indicated greater retention of 89Zr in the labeled cells in vivo than 111In. Conclusions: 89Zr-oxine stably binds many intracellular proteins and shows much higher and more stable protein binding than 111In-oxine. Intracellular protein binding of 89Zr accounts for the ability of 89Zr-oxine labeling to successfully track cells in vivo long-term on PET.
Keywords: cell labeling; cell tracking; positron emission tomography; protein labeling; radiolabeling; zirconium-89 oxine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
(89)Zr-Oxine Complex PET Cell Imaging in Monitoring Cell-based Therapies.Radiology. 2015 May;275(2):490-500. doi: 10.1148/radiol.15142849. Epub 2015 Feb 20. Radiology. 2015. PMID: 25706654 Free PMC article.
-
Tracking of NK Cells by Positron Emission Tomography Using 89Zr-Oxine Ex Vivo Cell Labeling.Methods Mol Biol. 2022;2463:153-161. doi: 10.1007/978-1-0716-2160-8_11. Methods Mol Biol. 2022. PMID: 35344173
-
Imaging of cell-based therapy using 89Zr-oxine ex vivo cell labeling for positron emission tomography.Nanotheranostics. 2021 Jan 1;5(1):27-35. doi: 10.7150/ntno.51391. eCollection 2021. Nanotheranostics. 2021. PMID: 33391973 Free PMC article. Review.
-
A kit formulation for the preparation of [89Zr]Zr(oxinate)4 for PET cell tracking: White blood cell labelling and comparison with [111In]In(oxinate)3.Nucl Med Biol. 2020 Nov-Dec;90-91:31-40. doi: 10.1016/j.nucmedbio.2020.09.002. Epub 2020 Sep 15. Nucl Med Biol. 2020. PMID: 32979725 Free PMC article.
-
PET of Adoptively Transferred Chimeric Antigen Receptor T Cells with 89Zr-Oxine.J Nucl Med. 2018 Oct;59(10):1531-1537. doi: 10.2967/jnumed.117.206714. Epub 2018 May 4. J Nucl Med. 2018. PMID: 29728514 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

