Osteogenic and Antibacterial Response of Levofloxacin-Loaded Mesoporous Nanoparticles Functionalized with N-Acetylcysteine
- PMID: 40284514
- PMCID: PMC12030205
- DOI: 10.3390/pharmaceutics17040519
Osteogenic and Antibacterial Response of Levofloxacin-Loaded Mesoporous Nanoparticles Functionalized with N-Acetylcysteine
Abstract
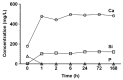
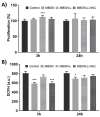
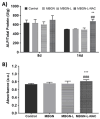
Background/Objectives: Bone infection is one of the most prevalent complications in orthopedic surgery. This pathology is mostly due to bacterial pathogens, among which S. aureus stands out. The formation of a bacterial biofilm makes systemic treatment with antibiotics ineffective. Herein we propose a nanosystem composed of mesoporous bioactive glass nanoparticles (MBGN) loaded with levofloxacin and functionalized with N-acetylcysteine (NAC), aiming to offer an alternative to current treatments. These nanoparticles would present antibacterial activity able to disintegrate the biofilm and regenerate the peri-implantar osseous tissue. Methods: MBGN of composition 82.5 SiO2-17.5 CaO have been synthesized, loaded with levofloxacin, and functionalized with NAC (MBGN-L-NAC). The antimicrobial activity against mature S. aureus biofilms and bioactivity of the nanosystem have been evaluated, as well as its biocompatibility and ability to promote murine pre-osteoblastic MC3T3-E1 differentiation. Results: MBGNs exhibited high surface areas and radial mesoporosity, allowing up to 23.1% (% w/w) of levofloxacin loading. NAC was covalently bound keeping the mucolytic thiol group, SH, available. NAC and levofloxacin combination enhances the activity against S. aureus by disrupting mature biofilm integrity. This nanosystem was biocompatible with pre-osteoblasts, enhanced their differentiation towards a mature osteoblast phenotype, and promoted bio-mimetic mineralization under in vitro conditions. MBGN-L-NAC nanoparticles induced greater osteogenic response of osteoprogenitor cells through increased alkaline phosphatase expression, increased mineralization, and stimulation of pre-osteoblast nodule formation. Conclusions: MBGN-L-NAC exhibits a more efficient antibacterial activity due to the biofilm disaggregation exerted by NAC, which also contributes to enhance the osteoinductive properties of MBGNs, providing a potential alternative to conventional strategies for the management of bone infections.
Keywords: N-acetylcysteine; bone infection; bone regeneration; levofloxacin; mesoporous bioactive nanoparticles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Dufour D., Leung V., Lévesque C.M. Bacterial Biofilm: Structure, Function, and Antimicrobial Resistance. Endod. Top. 2010;22:2–16. doi: 10.1111/j.1601-1546.2012.00277.x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

