The Role of Membrane-Bound Extracellular Vesicles During Co-Stimulation and Conjugation in the Ciliate Tetrahymena thermophila
- PMID: 40284639
- PMCID: PMC12029339
- DOI: 10.3390/microorganisms13040803
The Role of Membrane-Bound Extracellular Vesicles During Co-Stimulation and Conjugation in the Ciliate Tetrahymena thermophila
Abstract
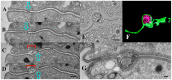
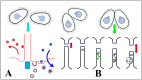
During sexual reproduction, the freshwater ciliate Tetrahymena thermophila sheds membrane-bound vesicles into the extracellular environment (cEMVs: ciliary extracellular micro-vesicles). We provide evidence that 100 nm vesicles shed from the cilia of starved cells promote mating between cells of complementary mating types. A proteomic analysis revealed that these EMVs are decorated with mating-type proteins expressed from the MAT locus, proteins that define a cell's sex (one of seven). Once the mating junction is established between cells, smaller 60 nm vesicles (junction vesicles) appear within the extracellular gap that separates mating partners. Junction vesicles (jEMVs) may play a role in remodeling the mating junction through which gametic pronuclei are exchanged. Evidence is presented demonstrating that cells must be able to internalize extracellular signals via some form of endocytosis in order to trigger conjugation. Finally, an evolutionarily conserved fusogen (Hap2) implicated in pore formation also appears necessary for jEMV processing. This system offers an excellent opportunity for studies on ectosome shedding, intercellular signaling and shed vesicle uptake by macro-pinocytosis, as they relate to sexual reproduction in the ciliate Tetrahymena thermophila.
Keywords: EMVs; Tetrahymena; ciliates; co-stimulation; conjugation; ectosomes; pheromones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Membrane dynamics at the nuclear exchange junction during early mating (one to four hours) in the ciliate Tetrahymena thermophila.Eukaryot Cell. 2015 Feb;14(2):116-27. doi: 10.1128/EC.00164-14. Epub 2014 Aug 8. Eukaryot Cell. 2015. PMID: 25107923 Free PMC article.
-
High frequency of sex and equal frequencies of mating types in natural populations of the ciliate Tetrahymena thermophila.Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8715-8. doi: 10.1073/pnas.92.19.8715. Proc Natl Acad Sci U S A. 1995. PMID: 7568003 Free PMC article.
-
Function of the male-gamete-specific fusion protein HAP2 in a seven-sexed ciliate.Curr Biol. 2014 Sep 22;24(18):2168-2173. doi: 10.1016/j.cub.2014.07.064. Epub 2014 Aug 21. Curr Biol. 2014. PMID: 25155508 Free PMC article.
-
Cilia-derived vesicles: An ancient route for intercellular communication.Semin Cell Dev Biol. 2022 Sep;129:82-92. doi: 10.1016/j.semcdb.2022.03.014. Epub 2022 Mar 26. Semin Cell Dev Biol. 2022. PMID: 35346578 Free PMC article. Review.
-
HAP2-Mediated Gamete Fusion: Lessons From the World of Unicellular Eukaryotes.Front Cell Dev Biol. 2022 Jan 7;9:807313. doi: 10.3389/fcell.2021.807313. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35071241 Free PMC article. Review.
References
-
- Orias E. Ciliate conjugation. In: Gall J.G., editor. The Molecular Biology of Ciliated Protozoa. Academic Press; London, UK: 1986. pp. 45–84.
-
- Cole E., Sugai T. Developmental progression of Tetrahymena through the cell cycle and conjugation. Methods Cell Biol. 2012;109:177–236. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

