Microbial Community Response and Assembly Process of Yellow Sand Matrix in a Desert Marginal Zone Under Morchella Cultivation
- PMID: 40284757
- PMCID: PMC12029328
- DOI: 10.3390/microorganisms13040921
Microbial Community Response and Assembly Process of Yellow Sand Matrix in a Desert Marginal Zone Under Morchella Cultivation
Abstract
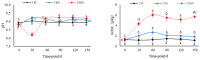
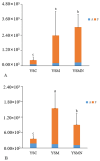
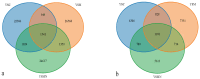
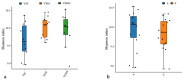
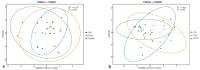
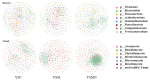
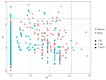
In this study, we investigated the adaptation of yellow-sand-substrate Morchella cultivation in the desert fringe and its effect on soil physicochemical properties and microbial communities. The qPCR and high-throughput sequencing with null modeling analyzed microbial diversity, networks, and assembly of Morchella cultivation under nutrient supplementation, linking physicochemical changes to microbial dynamics. The results showed that the yellow sand substrate can be planted with Morchella in the desert fringe area, as the Morchella cultivation with nutrient bags resulted in a yield of 691 g/m2 of Morchella fruit units. Cultivation of Morchella could significantly increase the physicochemical properties of the yellow sand substrate, such as soil organic matter (SOM), total nitrogen (TN), ammonium nitrogen (NH4+-N), and the microbial amount of carbon and nitrogen (MBC/MBN). The fungal community was dominated by Ascomycota, and Basidiomycota, Firmicutes, Bacteroidota, and Actinobacteriota. RDA analysis showed that Ascomycota and Proteobacteria were positively correlated with NH4+-N, MBN, SOM, MBC, acting potassium (AK), TN, and C/N. Morchella cultivation promoted a positive correlation-dominant microbial network pattern in the yellow sand substrate. The nutrient bag treatment reduced bacterial network complexity while enhancing fungal network complexity, connectivity and stability, accompanied by significant increases in Proteobacteria, Bacteroidota, Cladosporium, and Thermomyces relative abundances during cultivation until original substrate degradation. Deterministic processes dominated bacterial and fungal communities, and morel cultivation drove bacterial and fungal community assembly toward heterogeneous selection processes. The results of the study revealed the economic value of Morchella cultivation in the desert fringe and the application potential of improving the physicochemical properties of yellow sandy soil, which is of great importance for practical cultivation and application of morel mushrooms in the desert.
Keywords: Morchella; co-occurrence network; microbial community; yellow sand substrate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


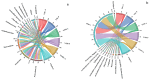
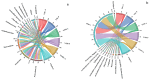
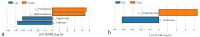

Similar articles
-
Enhancing Morchella Mushroom Yield and Quality Through the Amendment of Soil Physicochemical Properties and Microbial Community with Wood Ash.Microorganisms. 2024 Nov 23;12(12):2406. doi: 10.3390/microorganisms12122406. Microorganisms. 2024. PMID: 39770609 Free PMC article.
-
Effects of short-term application of Moutai lees biochar on nutrients and fungal community structure in yellow soil of Guizhou.Environ Sci Pollut Res Int. 2021 Dec;28(47):67404-67413. doi: 10.1007/s11356-021-15001-2. Epub 2021 Jul 12. Environ Sci Pollut Res Int. 2021. PMID: 34254242
-
Determination of the Effects of Pear-Morchella Intercropping Mode on M. sextelata Quality, Yield, and Soil Microbial Community.J Fungi (Basel). 2024 Nov 1;10(11):759. doi: 10.3390/jof10110759. J Fungi (Basel). 2024. PMID: 39590678 Free PMC article.
-
Fungal and bacterial community dynamics in substrates during the cultivation of morels (Morchella rufobrunnea) indoors.FEMS Microbiol Lett. 2019 Sep 1;366(17):fnz215. doi: 10.1093/femsle/fnz215. FEMS Microbiol Lett. 2019. PMID: 31603508 Free PMC article.
-
The Impact of Artificial Restoration of Alpine Grasslands in the Qilian Mountains on Vegetation, Soil Bacteria, and Soil Fungal Community Diversity.Microorganisms. 2024 Apr 25;12(5):854. doi: 10.3390/microorganisms12050854. Microorganisms. 2024. PMID: 38792684 Free PMC article. Review.
References
-
- Haider R., Agnello L., Shah S.M., Sufyan M., Khan N., Nazir A., Ciaccio M., Rehman S. Evaluating the antioxidant, anti-inflammatory, and neuroprotective potential of fruiting body and mycelium extracts from edible yellow morel (Morchella esculenta L. Pers.) J. Food Sci. 2025;90:e17619. doi: 10.1111/1750-3841.17619. - DOI - PMC - PubMed
-
- Ramya H., Ravikumar K.S., Fathimathu Z., Janardhanan K.K., Ajith T.A., Shah M.A., Farooq R., Reshi Z.A. Morel mushroom, Morchella from Kashmir Himalaya: A potential source of therapeutically useful bioactives that possess free radical scavenging, anti-inflammatory, and arthritic edema-inhibiting activities. Drug Chem. Toxicol. 2021;45:10–11. doi: 10.1080/01480545.2021.1894750. - DOI - PubMed
-
- Gao J., Fang D.L., Benard M.K., Chen X., Wu X., Du J.X., Yang Q., Chen H., Zheng H.H., An X.x., et al. Analysis of umami taste substances of morel mushroom (Morchella sextelata) hydrolysates derived from different enzymatic systems. Food Chem. 2021;362:130192. doi: 10.1016/j.foodchem.2021.130192. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

