Germination and Outgrowth of Bacillus subtilis Spores Deficient in BER and DisA Unveil Alternative Genetic Checkpoints
- PMID: 40284773
- PMCID: PMC12029834
- DOI: 10.3390/microorganisms13040939
Germination and Outgrowth of Bacillus subtilis Spores Deficient in BER and DisA Unveil Alternative Genetic Checkpoints
Abstract
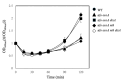
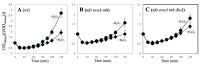
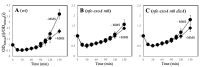
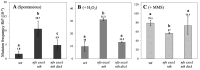
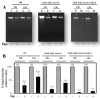
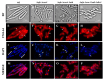
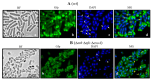
During Bacillus subtilis spore germination/outgrowth, the rehydration of the spore core and activation of aerobic metabolism can generate reactive oxygen species (ROS)-promoted DNA lesions that are repaired via the base excision repair pathway (BER). Accordingly, spores deficient in the AP-endonucleases (APEs) Nfo and ExoA exhibit a delayed outgrowth that is suppressed following disruption of the checkpoint protein DisA. Here, we report that DisA-independent DNA damage checkpoints operate during B. subtilis spore outgrowth. Consistent with this notion, spores lacking Nfo, ExoA, and Nth, which functions as an APE, did not suppress delayed outgrowth following disA disruption. Furthermore, in reference to the ∆nfo ∆exoA ∆nth spores, spores deficient for these APEs and DisA displayed a significantly higher number of oxidative genetic lesions and failed to properly segregate its chromosome during the first round of replication in the outgrowth stage. Finally, we found that DisA promotes low-fidelity repair and replication events, as revealed by DNA-alkaline gel electrophoresis (AGE) as well as spontaneous and H2O2-promoted RifR mutagenesis. Overall, our results unveil the existence of DisA-independent DNA damage checkpoint(s) that are activated by genomic lesions of an oxidative nature during spore germination and outgrowth, ensuring a proper transition to vegetative growth.
Keywords: BER; Bacillus subtilis; DisA; checkpoint; germination/outgrowth.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Pedraza-Reyes M., Abundiz-Yañez K., Rangel-Mendoza A., Martínez L.E., Barajas-Ornelas R.C., Cuéllar-Cruz M., Leyva-Sánchez H.C., Ayala-García V.M., Valenzuela-García L.I., Robleto E.A. Bacillus subtilis stress-associated mutagenesis and developmental DNA repair. Microbiol. Mol. Biol. Rev. 2024;88:e0015823. doi: 10.1128/mmbr.00158-23. - DOI - PMC - PubMed
-
- Paidhungat M., Setlow P. Spore germination and outgrowth. In: Hoch J.A., Losick R., editors. Bacillus subtilis and Its Relatives: From Genes to Cells. American Society for Microbiology; Washington, DC, USA: 2002. pp. 537–548.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

