Field Evaluation of a Ready-to-Use Porcine Circovirus Type 2 and Mycoplasma hyopneumoniae Vaccine in Naturally Infected Farms in Taiwan
- PMID: 40284806
- PMCID: PMC12031627
- DOI: 10.3390/vetsci12040304
Field Evaluation of a Ready-to-Use Porcine Circovirus Type 2 and Mycoplasma hyopneumoniae Vaccine in Naturally Infected Farms in Taiwan
Abstract
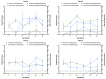
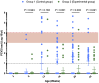
Porcine circovirus type 2 (PCV2) and Mycoplasma hyopneumoniae (MHP) are both important and common pathogens in the pig industry. Both pathogens are major contributors to the porcine respiratory disease complex and serve to potentiate other bacterial infections such as Actinobacillus pleuropneumonia. This study aims to evaluate the efficacy of a ready-to-use bivalent PCV2 and MHP vaccine in the field under naturally PCV2-infected farms against existing monovalent options. We evaluated PCV2 viremia, PCV2 antibodies, and lung lesion scores in slaughtered pigs in our study across four farms in Taiwan. Our results found that in two out of four farms, the piglets vaccinated with Porcilis® PCV M Hyo had superior whole-life PCV2 viremia reduction compared to the existing vaccination program on farms. In the lung lesion scoring, the Porcilis® PCV M Hyo group had significantly lower Actinobacillus pleuropneumonia-type lesions in pigs than in the competitor group in two out of three farms evaluated. In this field trial, Porcilis® PCV M Hyo proved to be efficacious in protecting piglets against both PCV2 viremia and the impact of MHP secondary infection, in the context of a reduction in viremia and reduced APP-like lesions found at slaughter.
Keywords: Actinobacillus pleuropneumoniae; Mycoplasma hyopneumoniae; bivalent vaccine; porcine circovirus type 2; porcine respiratory disease complex; swine; vaccination.
Conflict of interest statement
Intervet Animal Health Taiwan Ltd. is the sponsor of the project. S.-W.C. and B.-R.L. are employees of Intervet Animal Health Taiwan Ltd. However, the authors declare that the research was conducted with integrity, following all ethical guidelines. All other authors declare no competing interests.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

