Mitofusin-Mediated Mitochondrial Fusion Inhibits Pseudorabies Virus Infection in Porcine Cells
- PMID: 40284870
- PMCID: PMC12030837
- DOI: 10.3390/vetsci12040368
Mitofusin-Mediated Mitochondrial Fusion Inhibits Pseudorabies Virus Infection in Porcine Cells
Abstract
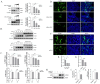
Background: Mitochondria are highly dynamic organelles that undergo fusion/fission dynamics, and emerging evidence has established that mitochondrial dynamics plays a crucial regulatory role in the process of viral infection. Nevertheless, the function of mitochondria dynamics during pseudorabies (PRV) infection remains uncertain. Methods: Our investigation commenced with examining PRV-induced alterations in mitochondrial dynamics, focusing on morphological changes and the expression levels of fusion/fission proteins. We then restored mitochondrial dynamics through Mfn1 (Mitofusin 1)/Mfn2 (Mitofusin 2) overexpression and mdivi-1 (mitochondrial division inhibitor-1) treatment to assess their impact on PRV replication and mitochondrial damage. Results: We found a downregulation of the mitochondrial fusion proteins Mfn1, Mfn2, and OPA1 (optic atrophy 1), along with the activation of the fission protein Drp-1 (dynamin-related protein 1) upon PRV infection. Restoring the function of mitochondrial fusion inhibited PRV infection. Furthermore, elevated mitochondrial membrane potential (MMP), decreased reactive oxygen species (ROS) levels, and an increased mitochondrial number were observed after overexpressing Mfns or treatment with mdivi-1. Conclusions: PRV infection impairs mitochondrial dynamics by altering mitochondrial fusion and fission proteins, and the promotion of Mfn-mediated mitochondrial fusion inhibits PRV replication.
Keywords: mitochondrial dynamics; mitochondrial fusion; mitofusin proteins; pseudorabies virus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

