Preparation and Evaluation of Novel Epitope-Based ETEC K88-K99 Bivalent Vaccine
- PMID: 40284883
- PMCID: PMC12030781
- DOI: 10.3390/vetsci12040381
Preparation and Evaluation of Novel Epitope-Based ETEC K88-K99 Bivalent Vaccine
Abstract
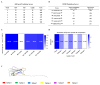
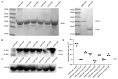
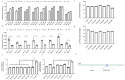
Enterotoxigenic Escherichia coli (ETEC) is one of the primary pathogens causing diarrhea in piglets, causing significant economic losses in the swine farming industry. Due to the numerous serotypes of ETEC, traditional vaccines fail to provide sufficient cross-protection, and subunit vaccines based on epitope design have emerged as a safer and more effective approach for prevention and control. Unlike vaccine development strategies that involve the tandem arrangement of multiple antigenic epitopes, this study used the K88-FaeG protein as a backbone and incorporated the antigenic epitopes of K99-FanC to achieve a better immunogenicity. By using bioinformatics software to predict B-cell linear epitopes (score of over 0.6), B-cell epitopes from three-dimensional structures (50% amino acid score of ≥0.2), and B-cell epitope IgG antibody subtypes, as well as docking analysis with Sus scrofa aminopeptidase N (APN) receptors, six antigenic epitopes of K99-FanC were selected. Through Western blotting and competitive ELISA, we confirmed that all six recombinant proteins exhibited binding capabilities to K88- and K99-positive serum. The ELISA results showed that the serum levels of specific IgG and IgA antibodies increased after immunization, with FaeG-Ep3 and FaeG-Ep5 inducing the highest antibody titers against FanC-IgG (Log2 = 14.96) and FaeG-IgG (Log2 = 17.96), respectively. Bacterial adhesion assays revealed that only FaeG-Ep3 effectively blocked the adhesion of both K99 and K88 to IPEC-J2 cells. Immunization challenge experiments showed that, in the unimmunized group, mice infected with K88 and K99 experienced weight loss (p < 0.05) with intestinal villus shedding and intestinal wall structural damage. However, in the FaeG-Ep3-immunized group, no significant weight loss occurred after infection, and the villus protection rate (83%) was the same as that in the FaeG and FanC immunized groups. Overall, the FaeG-Ep3 recombinant protein identified in this study shows potential vaccine application value and provides new insights for developing multivalent vaccines against ETEC.
Keywords: Enterotoxigenic Escherichia coli; FaeG; FanC; epitope; vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Hsueh F.C., Lin C.N., Chiou H.Y., Chia M.Y., Chiou M.T., Haga T., Kao C.F., Chang Y.C., Chang C.Y., Jeng C.R., et al. Updated phylogenetic analysis of the spike gene and identification of a novel recombinant porcine epidemic diarrhoea virus strain in Taiwan. Transbound. Emerg. Dis. 2020;67:417–430. doi: 10.1111/tbed.13365. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

