HTLV-1 p13 Protein Hijacks Macrophage Polarization and Promotes T-Cell Recruitment
- PMID: 40284913
- PMCID: PMC12031607
- DOI: 10.3390/v17040471
HTLV-1 p13 Protein Hijacks Macrophage Polarization and Promotes T-Cell Recruitment
Abstract
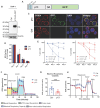
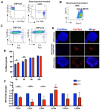
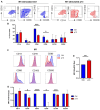
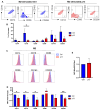
The human T-cell leukemia type-1 (HTLV-1) retrovirus establishes chronic life-long infection in a fraction of infected individuals associated with severe pathological conditions. Although the mechanism driving disease development is not fully understood, current evidence indicates the essential functions of viral regulatory proteins. Among these, the p13 protein has previously been shown to localize to the inner mitochondrial membrane in T cells, altering mitochondrial biology and T-cell function. While CD4+ T cells are the primary cell target of HTLV-1 infection, genomic viral DNA has also been detected in monocytes, macrophages, and dendritic cells, which orchestrate innate and adaptive immunity and play a critical role in protecting against virus-induce diseases by establishing the appropriate balance of pro and anti-inflammatory responses. Given the central role of mitochondria in monocyte differentiation, we investigated the effect of p13 in monocytes/macrophages and found that by localizing to mitochondria, p13 affects mitochondrial respiration. Moreover, we demonstrate that p13 expression affects macrophage polarization to favor the recruitment of CD4+ T cells, the primary target of the virus, potentially facilitating the spread of viral infection and the development of disease.
Keywords: ATLL; HAM/TSP; HTLV-1; HTLV-1 associated myelopathy/tropical spastic paraparesis; adult-T-cell leukemia/lymphoma; cytokines; macrophages; mitochondria; monocytes; p13; viral protein.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
High TCR Degeneracy Enhances Antiviral Efficacy of HTLV-1-Specific CTLs by Targeting Variant Viruses in HAM Patients.Int J Mol Sci. 2025 Jul 10;26(14):6602. doi: 10.3390/ijms26146602. Int J Mol Sci. 2025. PMID: 40724852 Free PMC article.
-
The role of human leukocyte antigen in HTLV-1 infection and progression to ATLL and HAM/TSP: a systematic review and meta-analysis.Virol J. 2025 Jan 20;22(1):13. doi: 10.1186/s12985-024-02612-7. Virol J. 2025. PMID: 39833815 Free PMC article.
-
Human T-Lymphotropic Virus (HTLV): Epidemiology, Genetic, Pathogenesis, and Future Challenges.Viruses. 2025 May 1;17(5):664. doi: 10.3390/v17050664. Viruses. 2025. PMID: 40431676 Free PMC article. Review.
-
HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP): Case based discussion of risk factors, clinical, and therapeutic considerations.J Neurol Sci. 2024 Apr 15;459:122973. doi: 10.1016/j.jns.2024.122973. Epub 2024 Mar 19. J Neurol Sci. 2024. PMID: 38520941 Review.
-
Coincidental Coexistence of Human T-lymphotropic Virus Type 1 (HTLV-1)-Associated Myelopathy/Tropical Spastic Paraparesis and Myasthenia Gravis in a Patient With Chronic HTLV-1 Infection: A Case Report.Cureus. 2025 Jul 17;17(7):e88163. doi: 10.7759/cureus.88163. eCollection 2025 Jul. Cureus. 2025. PMID: 40821191 Free PMC article.
References
-
- Poiesz B.J., Ruscetti F.W., Gazdar A.F., Bunn P.A., Minna J.D., Gallo R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

