The Clinical and Laboratory Landscape of COVID-19 During the Initial Period of the Pandemic and at the Beginning of the Omicron Era
- PMID: 40284924
- PMCID: PMC12031490
- DOI: 10.3390/v17040481
The Clinical and Laboratory Landscape of COVID-19 During the Initial Period of the Pandemic and at the Beginning of the Omicron Era
Abstract
Introduction: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) underwent significant mutations, resulting in the Omicron variant.
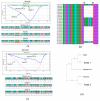
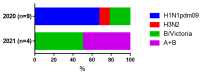
Methods: In this study, we analyzed blood samples from 98 patients with acute coronavirus disease 19 (COVID-19) hospitalized during the initial SARS-CoV-2 wave and the onset of Omicron in 2021. High-resolution melting (HRM) analysis of PCR products was used to analyze RNA extracted from clinical samples collected in July and November 2021 from patients infected with SARS-CoV-2.
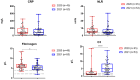
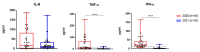
Results: HRM analysis revealed a characteristic deletion in the N protein RNA of the virus isolated in November 2021, associated with the Omicron variant. Elevated levels of inflammatory markers and interleukin-6 (IL-6) were observed in both waves of COVID-19. Complement levels and IgG and IgM antibodies to SARS-CoV-2 were detected more often during the second wave. An increase in hemagglutinin-inhibiting (HI) antibodies against influenza viruses was observed in paired blood specimens from moderate to severe COVID-19 patients during both outbreaks.
Conclusions: Patients admitted during both waves of COVID-19 showed a significant rise in inflammatory markers, suggesting that Omicron triggers inflammatory responses. The rapid formation of IgM and IgG in Omicron may indicate a faster immune response. Seasonal flu may negatively impact the clinical course of coronavirus infections.
Keywords: COVID-19; HRM analysis; N protein; Omicron; SARS-CoV-2; complement C3; influenza coinfections.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Akimkin V. COVID-19 epidemic process and evolution of SARS-CoV-2 genetic variants in the Russian Federation. Microbiol. Res. 2024;15:213–224. doi: 10.3390/microbiolres15010015. - DOI
-
- Meo S.A., Meo A.S., Al-Jassir F.F., Klonoff D.C. Omicron SARS-CoV-2 new variant: Global prevalence and biological and clinical characteristics. Europ. Rev. Med. Pharmacol. Sci. 2021;25:8012–8018. - PubMed
-
- Akimkin V.G., Popova A.Y., Khafizov K.F., Dubodelov D.V., Ugleva S.V., Semenenko T.A., Ploskireva A.A., Gorelov A.V., Pshenichnaya N.Y., Yezhlova E.B., et al. COVID-19: Evolution of the pandemic in Russia. Report II: Dynamics of the circulation of SARS-CoV-2 genetic variants. J. Microbiol. Epidemiol. Immunobiol. 2022;99:381–396. doi: 10.36233/0372-9311-295. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

