Role of Defective Interfering Particles in Complement-Mediated Lysis of Parainfluenza Virus-Infected Cells
- PMID: 40284931
- PMCID: PMC12031084
- DOI: 10.3390/v17040488
Role of Defective Interfering Particles in Complement-Mediated Lysis of Parainfluenza Virus-Infected Cells
Abstract
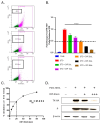
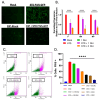
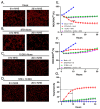
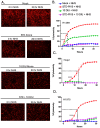
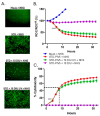
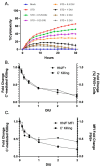
RNA viruses pose a significant global public health burden due to their high mutation rates, zoonotic potential, and ability to evade immune responses. A common aspect of their replication is the generation of defective interfering particles (DIPs), which contain truncated defective viral genomes (DVGs) that depend on full-length standard (STD) virus for replication. DVGs have gained recognition as they are increasingly detected in clinical samples from natural infections. While their role in modulating type I interferon (IFN-I) responses is well established, their impact on the complement (C') system is not understood. In this study, we examined how DVGs influence C'-mediated lysis during parainfluenza virus 5 (PIV5) infection using real-time in vitro cell viability assays. Our results demonstrated that C' effectively killed human lung epithelial cells infected with STD PIV5, whereas co-infection with DIP-enriched stocks significantly suppressed C'-mediated killing through mechanisms that were dependent on DVG replication but independent of IFN-I production. The titration of DI units in co-infection with STD PIV5 showed a strong linear relationship between DIP-mediated decreases in surface viral glycoprotein expression and the inhibition of C'-mediated lysis. Our findings reveal a previously unrecognized function of DVGs in modulating C' pathways, shedding light on their potential role in viral persistence and immune evasion.
Keywords: complement; defective interfering particles; defective viral genomes; interferon-beta; parainfluenza virus type 5.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Relationship Between Cell Surface Viral Glycoprotein Expression and Resistance of Parainfluenza Virus Persistently Infected Cells to Complement-Mediated Lysis.Pathogens. 2025 Aug 17;14(8):815. doi: 10.3390/pathogens14080815. Pathogens. 2025. PMID: 40872325 Free PMC article.
-
A MicroRNA Screen Identifies the Wnt Signaling Pathway as a Regulator of the Interferon Response during Flavivirus Infection.J Virol. 2017 Mar 29;91(8):e02388-16. doi: 10.1128/JVI.02388-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28148804 Free PMC article.
-
Resistance to complement-mediated lysis of parainfluenza virus 5-infected cells is acquired after transition from acute to persistent infection.J Virol. 2025 Feb 25;99(2):e0189524. doi: 10.1128/jvi.01895-24. Epub 2025 Jan 10. J Virol. 2025. PMID: 39791880 Free PMC article.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. Cochrane Database Syst Rev. 2011. PMID: 21735394
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003510. doi: 10.1002/14651858.CD003510.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2011 Jul 06;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. PMID: 17253490 Updated.
Cited by
-
A Type I IFN-Inducing Oncolytic Virus Improves NK Cell-Mediated Killing of Tumor Cells In Vitro Through Multiple Mechanisms.Viruses. 2025 Jun 25;17(7):897. doi: 10.3390/v17070897. Viruses. 2025. PMID: 40733515 Free PMC article.
-
Relationship Between Cell Surface Viral Glycoprotein Expression and Resistance of Parainfluenza Virus Persistently Infected Cells to Complement-Mediated Lysis.Pathogens. 2025 Aug 17;14(8):815. doi: 10.3390/pathogens14080815. Pathogens. 2025. PMID: 40872325 Free PMC article.
References
-
- Chakrabartty I., Khan M., Mahanta S., Chopra H., Dhawan M., Choudhary O.P., Bibi S., Mohanta Y.K., Emran T.B. Comparative overview of emerging RNA viruses: Epidemiology, pathogenesis, diagnosis and current treatment. Ann. Med. Surg. 2022;79:103985. doi: 10.1016/j.amsu.2022.103985. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

