In Silico and In Vitro Studies of the Approved Antibiotic Ceftaroline Fosamil and Its Metabolites as Inhibitors of SARS-CoV-2 Replication
- PMID: 40284934
- PMCID: PMC12031345
- DOI: 10.3390/v17040491
In Silico and In Vitro Studies of the Approved Antibiotic Ceftaroline Fosamil and Its Metabolites as Inhibitors of SARS-CoV-2 Replication
Abstract
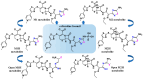
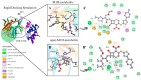
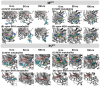
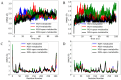
The SARS-CoV-2 proteases Mpro and PLpro are critical targets for antiviral drug development for the treatment of COVID-19. The 1,2,4-thiadiazole functional group is an inhibitor of cysteine proteases, such as papain and cathepsins. This chemical moiety is also present in ceftaroline fosamil (CF), an FDA-approved fifth-generation cephalosporin antibiotic. This study investigates the interactions between CF, its primary metabolites (M1 is dephosphorylated CF and M2 is an opened β-lactam ring) and derivatives (protonated M1H and M2H), and its open 1,2,4-thiadiazole rings derivatives (open-M1H and open-M2H) with SARS-CoV-2 proteases and evaluates CF's effects on in vitro viral replication. In silico analyses (molecular docking and molecular dynamics (MD) simulations) demonstrated that CF and its metabolites are potential inhibitors of PLpro and Mpro. Docking analysis indicated that the majority of the ligands were more stable with Mpro than PLpro; however, in vitro biochemical analysis indicated PLpro as the preferred target for CF. CF inhibited viral replication in the human Calu-3 cell model at submicromolar concentrations when added to cell culture medium at 12 h. Our results suggest that CF should be evaluated as a potential repurposing agent for COVID-19, considering not only viral proteases but also other viral targets and relevant cellular pathways. Additionally, the reactivity of sulfur in the 1,2,4-thiadiazole moiety warrants further exploration for the development of viral protease inhibitors.
Keywords: SARS-CoV-2; ceftaroline fosamil; cysteine proteases; drug repurposing; molecular docking; molecular dynamics; viral replication inhibition.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Mukherjee R., Dikic I. Proteases of SARS Coronaviruses. Encycl. Cell Biol. 2023;1:930
-
- Buttle D.J., Mort J.S. Encyclopedia of Biological Chemistry: Second Edition. Academic Press; Cambridge, MA, USA: 2013. Cysteine Proteases; pp. 589–592. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

