Nanotechnology-Driven Strategy Against SARS-CoV-2: Pluronic F127-Based Nanomicelles with or Without Atazanavir Reduce Viral Replication in Calu-3 Cells
- PMID: 40284961
- PMCID: PMC12031194
- DOI: 10.3390/v17040518
Nanotechnology-Driven Strategy Against SARS-CoV-2: Pluronic F127-Based Nanomicelles with or Without Atazanavir Reduce Viral Replication in Calu-3 Cells
Abstract
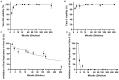
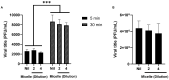
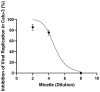
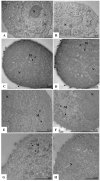
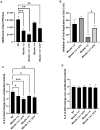
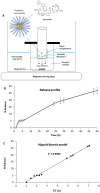
Despite extensive efforts, no highly effective antiviral molecule exists for treating moderate and severe COVID-19. Nanotechnology has emerged as a promising approach for developing novel drug delivery systems to enhance antiviral efficacy. Among these, polymeric nanomicelles improve the solubility, bioavailability, and cellular uptake of therapeutic agents. In this study, Pluronic F127-based nanomicelles were developed and evaluated for their antiviral activity against SARS-CoV-2. The nanomicelles, formulated using the direct dissolution method, exhibited an average size of 37.4 ± 8.01 nm and a polydispersity index (PDI) of 0.427 ± 0.01. Their antiviral efficacy was assessed in SARS-CoV-2-infected Vero E6 and Calu-3 cell models, where treatment with a 1:2 dilution inhibited viral replication by more than 90%. Cytotoxicity assays confirmed the nanomicelles were non-toxic to both cell lines after 72 h. In SARS-CoV-2-infected Calu-3 cells (human type II pneumocyte model), treatment with Pluronic F127-based nanomicelles containing atazanavir (ATV) significantly reduced viral replication, even under high MOI (2) and after 48 h, while also preventing IL-6 upregulation. To investigate their mechanism, viral pretreatment with nanomicelles showed no inhibitory effect. However, pre-exposure of Calu-3 cells led to significant viral replication reduction (>85% and >75% for 1:2 and 1:4 dilutions, respectively), as confirmed by transmission electron microscopy. These findings highlight Pluronic F127-based nanomicelles as a promising nanotechnology-driven strategy against SARS-CoV-2, reinforcing their potential for future antiviral therapies.
Keywords: Calu-3 cells; Pluronic F127; SARS-CoV-2; antiviral activity; atazanavir (ATV); nanotechnology; poloxamer; polymeric micelles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Direct pharmacological AMPK activation inhibits mucosal SARS-CoV-2 infection by reducing lipid metabolism, restoring autophagy flux and the type I IFN response.J Virol. 2025 Jul 22;99(7):e0039425. doi: 10.1128/jvi.00394-25. Epub 2025 Jun 12. J Virol. 2025. PMID: 40503889 Free PMC article.
-
SARS-CoV-2 infection enhancement by amphotericin B: implications for disease management.J Virol. 2025 Jul 22;99(7):e0051925. doi: 10.1128/jvi.00519-25. Epub 2025 Jun 4. J Virol. 2025. PMID: 40464579 Free PMC article.
-
The Ability of Combined Flavonol and Trihydroxyorganic Acid to Suppress SARS-CoV-2 Reproduction.Viruses. 2024 Dec 30;17(1):37. doi: 10.3390/v17010037. Viruses. 2024. PMID: 39861826 Free PMC article.
-
Nirmatrelvir combined with ritonavir for preventing and treating COVID-19.Cochrane Database Syst Rev. 2023 Nov 30;11(11):CD015395. doi: 10.1002/14651858.CD015395.pub3. Cochrane Database Syst Rev. 2023. PMID: 38032024 Free PMC article.
-
Ivermectin for preventing and treating COVID-19.Cochrane Database Syst Rev. 2022 Jun 21;6(6):CD015017. doi: 10.1002/14651858.CD015017.pub3. Cochrane Database Syst Rev. 2022. PMID: 35726131 Free PMC article.
References
-
- Mostafavi M., Shabani M. Leveraging Nanotechnology for Addressing COVID-19: Revealing Antiviral Approaches and Hurdles. World J. Nano Sci. Eng. 2024;14:1–14. doi: 10.4236/wjnse.2024.141001. - DOI
-
- Adekoya O.C., Adekoya G.J., Liu W., Sadiku E.R., Hamam Y. Pegylated Graphene Oxide For 4′-Fluorouridine Delivery: An Ab Initio Approach to Antiviral Therapy. Adv. Theory Simul. 2024;20:2401145. doi: 10.1002/adts.202401145. - DOI
-
- Yazdanian M., Memarnejadian A., Mahdavi M., Sadat S.M., Motevali F., Vahabpour R., Khanahmad H., Siadat S.D., Aghasadeghi M.R., Roohvand F. Immunization of Mice by BCG Formulated HCV Core Protein Elicited Higher Th1-Oriented Responses Compared to Pluronic-F127 Copolymer. Hepat. Mon. 2013;13:e14178. doi: 10.5812/hepatmon.14178. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- E-26/203.921/2024/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/201.426/2022/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/203.183/2023/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/203.628/2024/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/211.640/2021/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/210.136/2021/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- 88887.694990/2022-00/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- E-26/010.000981/2019/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- IOC-023-FIO-18-2-58/Instituto Oswaldo Cruz (IOC), Fiocruz, FIOTEC
- VPPIS-004-FIO-22-2-21/Programa INOVA Fiocruz
- 309790/2023-9/National Council for Scientific and Technological Development
- 312027/2022-2/National Council for Scientific and Technological Development
- 441922/2024-4/National Council for Scientific and Technological Development
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

