Modeling and Molecular Dynamics Studies of Flavone-DENV E-3 Protein-SWCNT Interaction at the Flavonoid Binding Sites
- PMID: 40284968
- PMCID: PMC12031533
- DOI: 10.3390/v17040525
Modeling and Molecular Dynamics Studies of Flavone-DENV E-3 Protein-SWCNT Interaction at the Flavonoid Binding Sites
Abstract
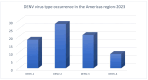
The DENV virus circulates freely in endemic regions and causes dengue disease. The vectors are Aedes aegypti and Aedes albopictus. The difficulties inherent in the nature of the DENV virus, its epidemiology, and its increasing incidence in recent years have led to the development of viable alternatives in the search for effective solutions for the treatment of this severe disease. Flavones such as tropoflavin, baicalein, and luteolin have anti-DENV activity. Molecular docking studies were performed between the flavones tropoflavin, baicalein, and luteolin and the DENV E-3 protein. Flavone-DENV E-3 complex interactions were analyzed at the flavonoid binding sites domain I of the B chain and domain II of the A chain reported in the literature. H-bond, π-π stacking, and π-cation interactions between flavones and the DENV E-3 protein at different binding energies were evaluated. Molecular dynamics studies for these interactions were performed to determine the molecular stability of the Flavone-DENV E-3 complexes. I also present here the results of the molecular interactions of the Flavone-DENV E-3-SWCNT complex. Due to recent advances in nanotechnology and their physicochemical properties, the utilization of nanoparticles such as SWCNT has increased in antiviral drug delivery.
Keywords: DENV E-3 protein; Flavone—DENV E-3 interactions; SWCNT–flavonoids; anti-DENV drugs; antiviral pharmacology; docking molecular; flavones; molecular dynamic simulation; nanomedicine; non-covalent interaction.
Conflict of interest statement
The author declares no conflicts of interest.
Figures

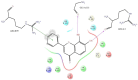
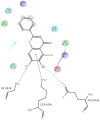
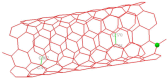
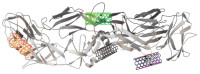

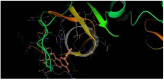
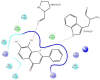
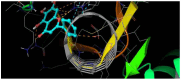
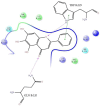
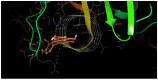
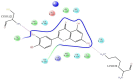
 H-bonds,
H-bonds,  hydrophobic,
hydrophobic,  Ionic,
Ionic,  Water bridge.
Water bridge.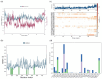
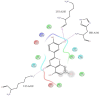
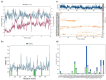
 H-bonds,
H-bonds,  hydrophobic,
hydrophobic,  Ionic,
Ionic,  Water bridge.
Water bridge.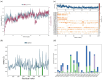
 H-bonds,
H-bonds,  hydrophobic,
hydrophobic,  Ionic,
Ionic,  Water bridge.
Water bridge.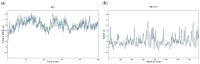
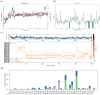
 H-bonds,
H-bonds,  hydrophobic,
hydrophobic,  Ionic,
Ionic,  Water bridge.
Water bridge.References
-
- Araújo J.M.G., Nogueira R.M.R., Schatzmayr H.G., Zanotto P.M., Gonzalo Bello G. Phylogeography and evolutionary history of dengue virus type. Infect. Genet. Evol. 2009;9:716–725. - PubMed
-
- World Health Organization Disease Outbreak News; Dengue—Global Situation. [(accessed on 21 December 2023)]. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON498.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

