Sulfatide Binds to Influenza B Virus and Enhances Viral Replication
- PMID: 40284974
- PMCID: PMC12031359
- DOI: 10.3390/v17040530
Sulfatide Binds to Influenza B Virus and Enhances Viral Replication
Abstract
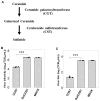
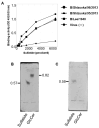
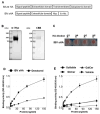
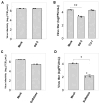
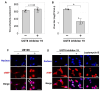
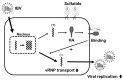
Seasonal influenza epidemics caused by influenza A viruses (IAV) and influenza B viruses (IBV) pose a substantial public health burden. Despite the significant impact of IBV, its restricted host range and the absence of documented pandemics have resulted in limited research attention relative to IAV. Understanding the viral infection mechanisms of both IAV and IBV is crucial for controlling seasonal epidemics. Previously, we demonstrated that 3'-O-sulfated galactosylceramide sulfatide binds to IAV and enhances viral replication, a finding with potential therapeutic implications. However, the role sulfatide plays in other influenza virus infections, including those caused by IBV, remains unknown. Accordingly, in this paper, we investigate the function of sulfatide during IBV infection. We demonstrate that sulfatide binds to IBV hemagglutinin (HA), and that sulfatide overexpression significantly enhances IBV replication, whereas treatment with sulfatase or an anti-sulfatide antibody markedly suppressed IBV replication. Moreover, further tests involving the inhibition of sulfatide biosynthesis resulted in the suppression of viral replication with impaired nuclear export of viral ribonucleoproteins (vRNPs). These findings establish that sulfatide is a critical regulator of IBV replication, which parallels its role in IAV infection, and suggest that targeting sulfatide-virus interactions can lead to broad-spectrum therapeutic strategies against influenza virus.
Keywords: antivirus therapeutics; hemagglutinin; influenza B virus; sulfatide; viral replication.
Conflict of interest statement
We have read the journal’s policy, and all authors of this manuscript declare that they have no conflicts of interest to disclose.
Figures
References
-
- Chang C.P., New A.E., Taylor J.F., Chiang H.S. Influenza virus isolations from dogs during a human epidemic in Taiwan. Int. J. Zoonoses. 1976;3:61–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

