Elevated Methylglyoxal: An Elusive Risk Factor Responsible for Early-Onset Cardiovascular Diseases in People Living with HIV-1 Infection
- PMID: 40284990
- PMCID: PMC12031240
- DOI: 10.3390/v17040547
Elevated Methylglyoxal: An Elusive Risk Factor Responsible for Early-Onset Cardiovascular Diseases in People Living with HIV-1 Infection
Abstract
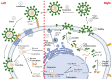
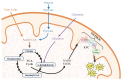
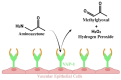
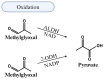
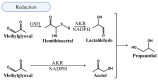
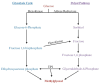
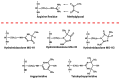
People living with HIV (PLWH) develop cardiovascular diseases (CVDs) about a decade earlier and at rates 2-3 times higher than the general population. At present, pharmacological strategies to delay the onset of CVDs in PLWH are unavailable, in part because of an incomplete understanding of its molecular causes. We and others recently uncovered elevated levels of the toxic glycolysis and inflammation-induced byproduct methylglyoxal (MG) in plasma from PLWH and from HIV-infected humanized mice (Hu-mice). We also found a reduction in expression of the primary MG-degrading enzyme glyoxalase I (Glo-I) in autopsied cardiac tissues from HIV-1-infected individuals and HIV-1-infected Hu-mice. Increasing the expression of Glo-I in HIV-1-infected Hu-mice not only attenuated heart failure but also reduced endothelial cell damage, increased the density of perfused microvessels, prevented microvascular leakage and micro-ischemia, and blunted the expression of the inflammation-induced protein vascular protein-1 (VAP-1), key mediators of CVDs. In this narrative review, we posit that elevated MG is a contributing cause for the early onset of CVDs in PLWH. Pharmacological strategies to prevent MG accumulation and delay the development of early-onset CVDs in PLWH are also discussed.
Keywords: HIV-1; aldehyde dehydrogenase; aldo-keto reductase; antioxidant transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2); cardiovascular diseases; glutathione; glyoxalase-I; methylglyoxal; nicotinamide adenine dinucleotide; nicotinamide adenine dinucleotide phosphate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
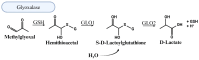

Similar articles
-
Identification of glyoxalase A in group B Streptococcus and its contribution to methylglyoxal tolerance and virulence.Infect Immun. 2025 Apr 8;93(4):e0054024. doi: 10.1128/iai.00540-24. Epub 2025 Feb 26. Infect Immun. 2025. PMID: 40008888 Free PMC article.
-
Antidepressants for depression in adults with HIV infection.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD008525. doi: 10.1002/14651858.CD008525.pub3. Cochrane Database Syst Rev. 2018. PMID: 29355886 Free PMC article.
-
Diastolic Dysfunction with Vascular Deficits in HIV-1-Infected Female Humanized Mice Treated with Antiretroviral Drugs.Int J Mol Sci. 2025 Apr 17;26(8):3801. doi: 10.3390/ijms26083801. Int J Mol Sci. 2025. PMID: 40332423 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Mobile phone text messaging for medication adherence in secondary prevention of cardiovascular disease.Cochrane Database Syst Rev. 2024 Mar 27;3(3):CD011851. doi: 10.1002/14651858.CD011851.pub3. Cochrane Database Syst Rev. 2024. PMID: 38533994 Free PMC article.
References
-
- Grobler A., Cawood C., Khanyile D., Puren A., Kharsany A.B. Progress of UNAIDS 90-90-90 targets in a district in KwaZulu-Natal, South Africa, with high HIV burden, in the HIPSS study: A household-based complex multilevel community survey. Lancet HIV. 2017;4:e505–e513. doi: 10.1016/S2352-3018(17)30122-4. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

