Inactivated Viral Vaccine BBV87 Protects Against Chikungunya Virus Challenge in a Non-Human Primate Model
- PMID: 40284993
- PMCID: PMC12031606
- DOI: 10.3390/v17040550
Inactivated Viral Vaccine BBV87 Protects Against Chikungunya Virus Challenge in a Non-Human Primate Model
Abstract
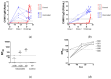
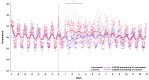
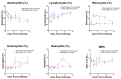
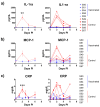
Chikungunya virus (CHIKV) is an alphavirus transmitted by mosquitos that poses a threat to global public health and for which there is an urgent need for widespread access to globally licensed vaccines. Here, we demonstrate that an inactivated CHIKV vaccine (BBV87) protects against systemic infection with CHIKV in a non-human primate (NHP) challenge model. Groups of five cynomolgus macaques received two doses of 20 µg BBV87 vaccine or saline alone (28 days apart). Twenty-eight days after the second immunisation, all animals were challenged with CHIKV. All controls were productively infected with detectable viremia and pathological responses following challenge, including altered thermoregulation, haematological and cytokine changes. Critically, the histopathological analysis of finger joints identified areas of inflammation in the synovium. By contrast vaccinated macaques had no detectable viremia and none of the pathological changes were reported in control animals. This study demonstrates that a 20 µg dose of BBV87 vaccine confers robust protection in vivo, both on the acquisition of infection and pathology.
Keywords: chikungunya virus; immunity; non-human primate; pathogenesis; vaccine.
Conflict of interest statement
Author Sumathy Kandaswamy was employed by the company Bharat Biotech International Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Humoral and cellular immune response to a single dose of a novel bivalent recombinant adenovirus-vector vaccine against West Nile virus and chikungunya virus in mice.Virol J. 2025 Jul 25;22(1):256. doi: 10.1186/s12985-025-02878-5. Virol J. 2025. PMID: 40713773 Free PMC article.
-
Generation and characterization of infectious clones of chikungunya virus from an Indian strain as a resource towards chikungunya vaccine research.Virus Res. 2025 Jun;356:199571. doi: 10.1016/j.virusres.2025.199571. Epub 2025 Apr 9. Virus Res. 2025. PMID: 40216162 Free PMC article.
-
Combined immunogenicity evaluation for a new single-dose live-attenuated chikungunya vaccine.J Travel Med. 2024 Oct 19;31(7):taae084. doi: 10.1093/jtm/taae084. J Travel Med. 2024. PMID: 38959854 Clinical Trial.
-
From bench to clinic: the development of VLA1553/IXCHIQ, a live-attenuated chikungunya vaccine.J Travel Med. 2024 Oct 19;31(7):taae123. doi: 10.1093/jtm/taae123. J Travel Med. 2024. PMID: 39255380 Free PMC article. Review.
-
Evidence of previous but not current transmission of chikungunya virus in southern and central Vietnam: Results from a systematic review and a seroprevalence study in four locations.PLoS Negl Trop Dis. 2018 Feb 9;12(2):e0006246. doi: 10.1371/journal.pntd.0006246. eCollection 2018 Feb. PLoS Negl Trop Dis. 2018. PMID: 29425199 Free PMC article.
References
-
- Arankalle V.A., Shrivastava S., Cherian S., Gunjikar R.S., Walimbe A.M., Jadhav S.M., Sudeep A.B., Mishra A.C. Genetic divergence of Chikungunya viruses in India (1963–2006) with special reference to the 2005–2006 explosive epidemic. Pt 7J. Gen. Virol. 2007;88:1967–1976. doi: 10.1099/vir.0.82714-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

