A No-Brainer! The Therapeutic Potential of TRIM Proteins in Viral and Central Nervous System Diseases
- PMID: 40285004
- PMCID: PMC12031127
- DOI: 10.3390/v17040562
A No-Brainer! The Therapeutic Potential of TRIM Proteins in Viral and Central Nervous System Diseases
Abstract
Tripartite motif (TRIM) proteins comprise an important class of E3 ubiquitin ligases that regulate numerous biological processes including protein expression, cellular signaling pathways, and innate immunity. This ubiquitous participation in fundamental aspects of biology has made TRIM proteins a focus of study in many fields and has illuminated the negative impact they exert when functioning improperly. Disruption of TRIM function has been linked to the success of various pathogens and separately to the occurrence and development of several neurodegenerative diseases, making TRIM proteins an appealing candidate to study for novel therapeutic approaches. Here, we review the current findings on TRIM proteins that demonstrate their analogous properties in the distinct fields of viral infection and central nervous system (CNS) disorders. We also examine recent advancements in drug development and targeted protein degradation as potential strategies for TRIM-mediated therapeutic treatments and discuss the implications these technologies have on future research directions.
Keywords: CNS diseases; E3 ubiquitin ligase; PROTAC; disease therapeutic; innate immunity; protein degradation; trim-away; tripartite motif (TRIM); ubiquitin; virus infection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
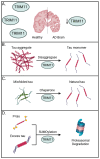
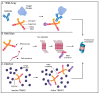
Similar articles
-
The Human Papillomavirus E6 Oncoprotein Targets USP15 and TRIM25 To Suppress RIG-I-Mediated Innate Immune Signaling.J Virol. 2018 Feb 26;92(6):e01737-17. doi: 10.1128/JVI.01737-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29263274 Free PMC article.
-
Emerging Roles of TRIM56 in Antiviral Innate Immunity.Viruses. 2025 Jan 7;17(1):72. doi: 10.3390/v17010072. Viruses. 2025. PMID: 39861861 Free PMC article. Review.
-
Uncommon Non-MS Demyelinating Disorders of the Central Nervous System.Curr Neurol Neurosci Rep. 2025 Jul 1;25(1):45. doi: 10.1007/s11910-025-01432-8. Curr Neurol Neurosci Rep. 2025. PMID: 40591029 Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Equine lentivirus Gag protein degrades mitochondrial antiviral signaling protein via the E3 ubiquitin ligase Smurf1.J Virol. 2025 Jan 31;99(1):e0169124. doi: 10.1128/jvi.01691-24. Epub 2024 Dec 12. J Virol. 2025. PMID: 39665545 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

