3D Mitochondrial Structure in Aging Human Skeletal Muscle: Insights Into MFN-2-Mediated Changes
- PMID: 40285369
- PMCID: PMC12266761
- DOI: 10.1111/acel.70054
3D Mitochondrial Structure in Aging Human Skeletal Muscle: Insights Into MFN-2-Mediated Changes
Abstract
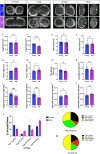
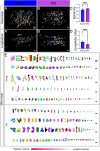
Age-related skeletal muscle atrophy, known as sarcopenia, is characterized by loss of muscle mass, strength, endurance, and oxidative capacity. Although exercise has been shown to mitigate sarcopenia, the underlying governing mechanisms are poorly understood. Mitochondrial dysfunction is implicated in aging and sarcopenia; however, few studies explore how mitochondrial structure contributes to this dysfunction. In this study, we sought to understand how aging impacts mitochondrial three-dimensional (3D) structure and its regulators in skeletal muscle. We hypothesized that aging leads to remodeling of mitochondrial 3D architecture permissive to dysfunction and is ameliorated by exercise. Using serial block-face scanning electron microscopy (SBF-SEM) and Amira software, mitochondrial 3D reconstructions from patient biopsies were generated and analyzed. Across five human cohorts, we correlate differences in magnetic resonance imaging, mitochondria 3D structure, exercise parameters, and plasma immune markers between young (under 50 years) and old (over 50 years) individuals. We found that mitochondria are less spherical and more complex, indicating age-related declines in contact site capacity. Additionally, aged samples showed a larger volume phenotype in both female and male humans, indicating potential mitochondrial swelling. Concomitantly, muscle area, exercise capacity, and mitochondrial dynamic proteins showed age-related losses. Exercise stimulation restored mitofusin 2 (MFN2), one such of these mitochondrial dynamic proteins, which we show is required for the integrity of mitochondrial structure. Furthermore, we show that this pathway is evolutionarily conserved, as Marf, the MFN2 ortholog in Drosophila, knockdown alters mitochondrial morphology and leads to the downregulation of genes regulating mitochondrial processes. Our results define age-related structural changes in mitochondria and further suggest that exercise may mitigate age-related structural decline through modulation of mitofusin 2.
Keywords: 3D reconstruction; MFN‐2; aging; exercise; human skeletal muscle; mitochondria.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Update of
-
3D Mitochondrial Structure in Aging Human Skeletal Muscle: Insights into MFN-2 Mediated Changes.bioRxiv [Preprint]. 2024 Jun 8:2023.11.13.566502. doi: 10.1101/2023.11.13.566502. bioRxiv. 2024. Update in: Aging Cell. 2025 Jul;24(7):e70054. doi: 10.1111/acel.70054. PMID: 38168206 Free PMC article. Updated. Preprint.
References
-
- Baar, K. , Wende A. R., Jones T. E., et al. 2002. “Adaptations of Skeletal Muscle to Exercise: Rapid Increase in the Transcriptional Coactivator PGC‐1.” FASEB Journal 16: 1879–1886. - PubMed
-
- Basso, V. , Marchesan E., Peggion C., et al. 2018. “Regulation of ER‐Mitochondria Contacts by Parkin via Mfn2.” Pharmacological Research 138: 43–56. - PubMed
MeSH terms
Substances
Grants and funding
- UL1 TR000445/TR/NCATS NIH HHS/United States
- MCB1955975/NSF
- 1S10OD012324-01/Vanderbilt Cell Imaging Shared Resource
- S10RR025497/GF/NIH HHS/United States
- HD090061/GF/NIH HHS/United States
- R01DK-133698/GF/NIH HHS/United States
- 5I01BX005352-04/Department of Veterans Affairs Office of Research Award
- UL1 RR024975/RR/NCRR NIH HHS/United States
- EES2112556/NSF
- MD007586/GF/NIH HHS/United States
- 1022355/Burroughs Wellcome Fund
- R21 TW012635/TW/FIC NIH HHS/United States
- CA163069/GF/NIH HHS/United States
- U54 CA163069/CA/NCI NIH HHS/United States
- UL1 TR000445-06/TR/NCATS NIH HHS/United States
- 2011577/NSF
- R21TW012635/GF/NIH HHS/United States
- DK007563/GF/NIH HHS/United States
- G12 MD007586/MD/NIMHD NIH HHS/United States
- 1S10OD021630-01/Vanderbilt Cell Imaging Shared Resource
- ANRF/IRG/2024/001777/LS/ANRF (Anusandhan National Research Foundation)
- R21DK119879/GF/NIH HHS/United States
- DK020593/Vanderbilt Diabetes Research and Training Center, Vanderbilt University Medical Center
- R01 HD090061/HD/NICHD NIH HHS/United States
- S10 RR025497/RR/NCRR NIH HHS/United States
- EES1817282/NSF
- ANRF/ECRG/2024/001042/LS/ANRF
- U54 MD007586/MD/NIMHD NIH HHS/United States
- R21 DK119879/DK/NIDDK NIH HHS/United States
- 1021868.01/Burroughs Wellcome Fund
- S10 OD021630/OD/NIH HHS/United States
- 5R25HL106365-12/GF/NIH HHS/United States
- R25 HL106365/HL/NHLBI NIH HHS/United States
- NFSG/IISER-Tirupati
- 24IVPHA1297559/American Heart Association
- UL1 RR024975-01/RR/NCRR NIH HHS/United States
- T32 DK101003/DK/NIDDK NIH HHS/United States
- 2022-253529/Chan Zuckerberg Initiative
- I01 BX005352/BX/BLRD VA/United States
- 5P30CA068485-29/Vanderbilt Cell Imaging Shared Resource
- P30 DK020593/DK/NIDDK NIH HHS/United States
- 5UL1TR002243-03/Vanderbilt Institute for Clinical and Translational Research Program
- P30 CA068485/CA/NCI NIH HHS/United States
- Silicon Valley Community Foundation
- United Negro College Fund (UNCF)/Bristol-Myers Squibb E.E. Just Faculty Fund
- American Society of Nephrology KidneyCure Transition to Independence Grant, ASN Foundation
- UL1 TR002243/TR/NCATS NIH HHS/United States
- 16SDG27080009/American Heart Association
- T32 DK007563/DK/NIDDK NIH HHS/United States
- S10 OD012324/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

