Anchored Indirect Treatment Comparison Finds Comparable Effects of Pegcetacoplan and Iptacopan in Paroxysmal Nocturnal Haemoglobinuria
- PMID: 40285403
- PMCID: PMC12224562
- DOI: 10.1111/ejh.14422
Anchored Indirect Treatment Comparison Finds Comparable Effects of Pegcetacoplan and Iptacopan in Paroxysmal Nocturnal Haemoglobinuria
Abstract
Background: Paroxysmal nocturnal haemoglobinuria (PNH) is an ultra-rare, acquired non-malignant haematological disorder characterised by thrombosis risk, serious complications and debilitating symptoms in untreated patients.
Objective: This anchored indirect treatment comparison (ITC) evaluated efficacy data between proximal complement 3 inhibitor (C3i) pegcetacoplan and factor B inhibitor, iptacopan, in patients with PNH previously treated with complement 5 inhibitors (C5i; eculizumab, ravulizumab).
Methods: Respective pivotal studies provided patient-level trial data for pegcetacoplan (16-week PEGASUS [NCT03500549]) and published data for iptacopan (24-week APPLY PNH [NCT04558918]). Differences in study design, duration and statistical methods between PEGASUS and APPLY PNH necessitated the comparative analyses to be conducted on secondary measures based on haemoglobin (Hb) levels, absolute reticulocyte count (ARC), lactate dehydrogenase (LDH) levels, and patient-reported outcomes on the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) scale scores. The availability of a common reference C5i treatment group in both PEGASUS and APPLY PNH studies allowed anchored ITC (Bucher method). Simulated treatment comparison (STC) assessed the robustness of the main analysis.
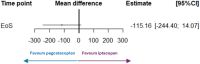
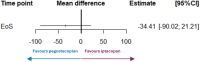
Results: Overall, baseline characteristics of the populations in the PEGASUS and APPLY PNH studies were broadly comparable. Anchored ITC showed comparable outcomes (mean difference, [95% CI]) on change-from-baseline to end of study for pegcetacoplan versus C5i, and iptacopan versus C5i, respectively, across endpoints: Hb level (-0.49 g/dL [-1.78, 0.80]); ARC (-34.41 × 109/L [-90.02, 21.21]); LDH level (-115.16 U/L [-244.40, 14.01]); FACIT-Fatigue score (3.57 [-5.60, 12.73]). Finally, the STC produced results consistent with the main Bucher analyses across all clinical endpoints and patient-reported fatigue, providing similar point estimates and confidence intervals.
Conclusion: This anchored ITC, based on data from pivotal trials, did not indicate significant differences in clinical or patient-reported outcomes between pegcetacoplan and iptacopan in PNH treatment. The findings suggest that PNH treatment decisions should also consider individualised disease- and patient-related factors.
Trial registration: ClinicalTrials.gov identifier: NCT03500549.
Keywords: indirect treatment comparison; iptacopan; paroxysmal nocturnal haemoglobinuria; pegcetacoplan.
© 2025 The Author(s). European Journal of Haematology published by John Wiley & Sons Ltd.
Conflict of interest statement
Koo Wilson, Zalmai Hakimi, Jameel Nazir and Barbara Czech are employed by Sobi, the sponsors of the study. Regis Peffault de Latour is an expert consultant/speaker for Alexion, Amgen, Apellis, Jazz, Novartis, Pfizer, Roche and Samsung, and received research grants from Alexion, Novartis and Pfizer. Austin G. Kulasekararaj reports consultancy fees/honoraria/speaker's bureau fees from Achillion, Akari, Alexion, AstraZeneca Rare Disease, Amgen, Apellis, Biocryst, Celgene, F. Hoffmann‐La Roche, Novartis, Pfizer, NovoNordisk, Samsung, Silence Therapeutics and Ra Pharma and research funding from Celgene/BMS and Novartis. Piotr Wojciechowski is employed by Clever‐Access (formerly Assignity), the company received funding to conduct the analysis of this study.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

